Histopathological Variants of Hepatocellular Carcinomas: an Update According to the 5th Edition of the WHO Classification of Digestive System Tumors
Article information
Abstract
Hepatocellular carcinoma (HCC) is heterogeneous in pathogenesis, phenotype and biological behavior. Various histopathological features of HCC had been sporadically described, and with the identification of common molecular alterations of HCC and its genomic landscape over the last decade, morpho-molecular correlation of HCC has become possible. As a result, up to 35% of HCCs can now be classified into histopathological variants, many of which have unique molecular characteristics. This review will provide an introduction to the variously described histopathological variants of HCC in the updated WHO Classification of Digestive System Tumors.
INTRODUCTION
Hepatocellular carcinoma (HCC) is defined as a primary hepatic malignancy showing hepatocellular differentiation, and it accounts for 75-85% of primary liver cancers [1]. HCCs mostly arise in a background of chronic liver disease, the most common etiologies being hepatitis B, hepatitis C, chronic alcohol abuse, non-alcoholic fatty liver disease, inherited diseases (e.g. hemochromatosis and glycogen storage disease), and exogenous substances, such as aflatoxin B1 [1,2]. A minority of HCCs develop in a background of normal or near-normal liver, the most common setting being HCCs arising in hepatocellular adenomas [1,3,4].
Histologically, conventional HCCs demonstrate hepatocytic differentiation (i.e., the tumor cells resemble the appearance of hepatocytes with varying degrees of cyto-architectural atypia). The typical HCC tumor cells are cuboidal in shape, contain abundant eosinophilic cytoplasm with centrally located nuclei, and are frequently arranged in a trabecular pattern of variable thickness that at least vaguely recapitulates the trabecular architecture of the normal hepatic acinus, with very little intratumoral stroma (Fig. 1) [5]. However, many cases demonstrate deviations from this pattern; for example, some HCCs are predominantly composed of tumor cells with clear cytoplasm, some may show a predominantly pseudoglandular architecture and some may show abundant intratumoral fibrous stroma.
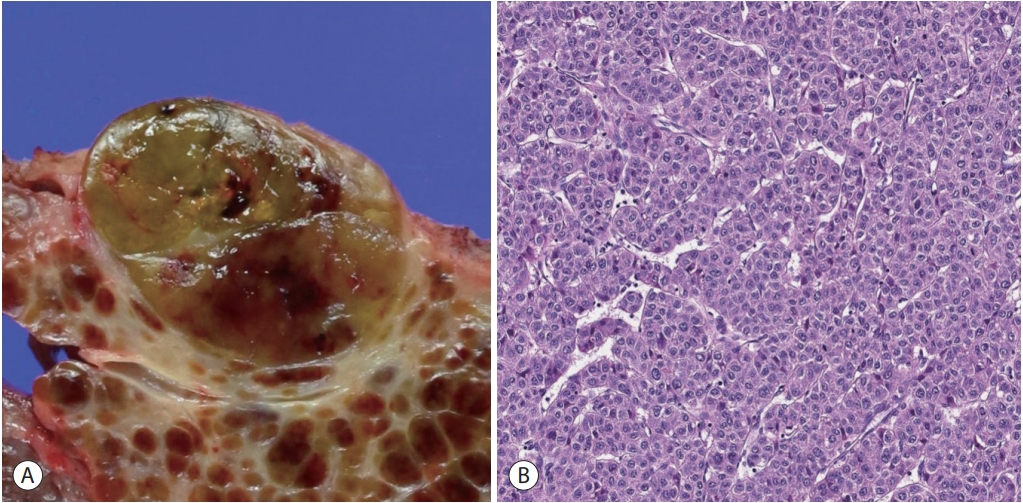
Hepatocellular carcinoma, conventional. (A) An expanding nodular tumor with a slightly bulging cut surface is seen. The tumor is slightly bile-tinged with small foci of hemorrhage. (B) Microscopic examination of a typical hepatocellular carcinoma shows tumor cells with eosinophilic cytoplasm arranged in a trabecular pattern (hematoxylin-eosin stain, ×200).
The 4th Edition of the World Health Organization (WHO) Classification of Digestive System Tumors (2010) described the histopathological features of HCC in detail, including detailed descriptions of “cytological variants”, such as pleomorphic cells, clear cells and spindle cells [6]. In addition, a few “special types” of HCC were recognized, including fibrolamellar carcinoma, scirrhous HCC, undifferentiated carcinoma, lymphoepithelioma-like carcinoma and sarcomatoid HCC. At that time, these special types were based mostly on the morphological features of the tumor.
Since then, numerous large-scale genomic analyses in the recent years have clarified the mutational landscape and identified key cell-signaling and metabolic pathways related to hepatocarcinogenesis [7-10]. This has resulted in a large number of molecular subclasses and signatures of HCC, and has highlighted the heterogeneity of HCC, in pathogenesis, phenotype and biological behavior. Recent studies have provided increasing evidence for morpho-molecular correlation of HCCs, and these efforts have resulted in several proposed histopathological variants of HCC [11]. As many as 35% of HCCs can now be classified into histopathological variants, which have been introduced in the latest WHO Classification of Digestive System Tumors, 5th Edition [1] and the distinction between most of these variants is now supported by the molecular features of the tumors (Table 1). In this review, we will summarize the recently described histopathological variants of HCCs.
STEATOHEPATITIC VARIANT
The steatohepatitic variant of HCC, or steatohepatitic HCC, demonstrates the histological features of steatohepatitis within the tumor, including steatosis, ballooning of tumor cells, inflammation and the typical “chicken-wire pattern” pericellular fibrosis (Fig. 2) [11-14]. It accounts for 5-20% of HCCs, and the background liver may show steatohepatitis [13,14]. Although this variant has been shown to be less often associated with vascular invasion or satellite nodules, its prognosis seems to be similar to conventional HCCs so far [11]. The key molecular features include IL-6/JAK/STAT activation, and lower frequency of CTNNB1 , TERT , and TP53 mutations compared to other HCCs [11].
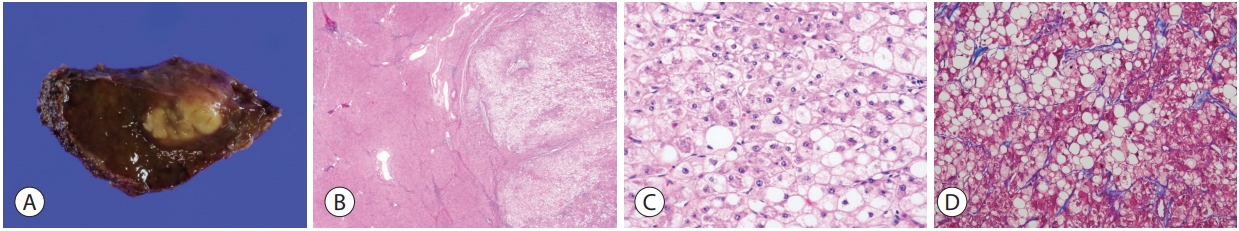
Hepatocellular carcinoma, steatohepatitic variant. (A) The tumor shows a yellowish hue on gross examination due to the lipid content. (B) At low power magnification, there is extensive intratumoral steatosis (right, hematoxylin-eosin stain, ×12.5). (C) Higher power magnification demonstrates the histological features of steatohepatitis, including steatosis, hepatocyte ballooning and Mallory-Denk bodies (hematoxylineosin stain, ×100). (D) The perisinusoidal “chicken-wire pattern” fibrosis is highlighted by Masson’s trichrome stain (×100).
CLEAR CELL VARIANT
In the clear cell variant of HCC, the majority (>80%) of tumor cells demonstrate clear cytoplasm (Fig. 3) [15]. The clear cytoplasm is a result of glycogen accumulation, although it is sometimes hard to discriminate from lipid droplets that are intermixed; in this regard, some degree of steatosis is acceptable for a diagnosis of clear cell HCC. About 3-7% of HCCs show this morphology, and although the prognosis has been shown to be better compared to conventional HCCs, there is still a lack of information on the clinical correlates or the molecular features of these tumors. Of note, it is important to discriminate clear cell HCC from metastatic clear cell renal cell carcinoma, as the morphology may be remarkably similar. Therefore, on encountering such cases in a biopsy, it is advisable to check the patient’s clinicoradiological information, and to consider performing immunohistochemical stains (e.g., PAX-8, CD10, RCC for renal cell carcinoma, and hepatocellular differentiation markers such as HepPar-1 and arginase-1 for HCC) to avoid this diagnostic pitfall [16].
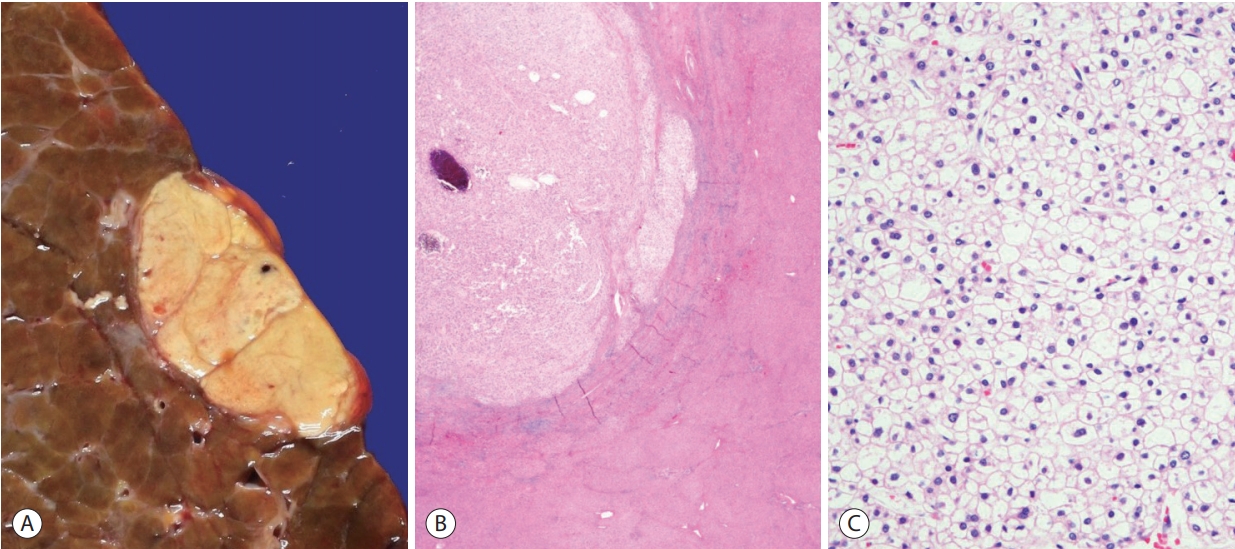
Hepatocellular carcinoma, clear cell variant. (A) Gross feature of the tumor. (B) The tumor (upper left) appears lighter compared to the adjacent hepatic parenchyma at low power (hematoxylin-eosin stain, ×12.5). (C) Most of the tumor cells show clear cytoplasm, due to intracytoplasmic glycogen accumulation (hematoxylin-eosin stain, ×200).
MACROTRABECULAR-MASSIVE VARIANT
The macrotrabecular-massive variant of HCC demonstrates a characteristic microscopic appearance: prominent thick trabeculae, measuring more than 6-10 cells in thickness, in at least 50% of the tumor (Fig. 4) [1,11,17]. The relative frequency of this variant has been described to be about 5% in the WHO classification, although some have reported frequencies of up to 20%, probably as a result of the varying histological criteria; the trabecular thickness criteria for macrotrabecular- massive HCC has varied from 6 to 20 cells thick in the literature. From the molecular level, this variant is characterized by frequent TP53 mutation and FGF19 amplification [11,17]. Macrotrabecular-massive HCCs have also been associated with frequent HBV infection, frequent vascular invasion, poor differentiation, CK19 expression, high serum AFP levels and a poor clinical outcome [11,17].
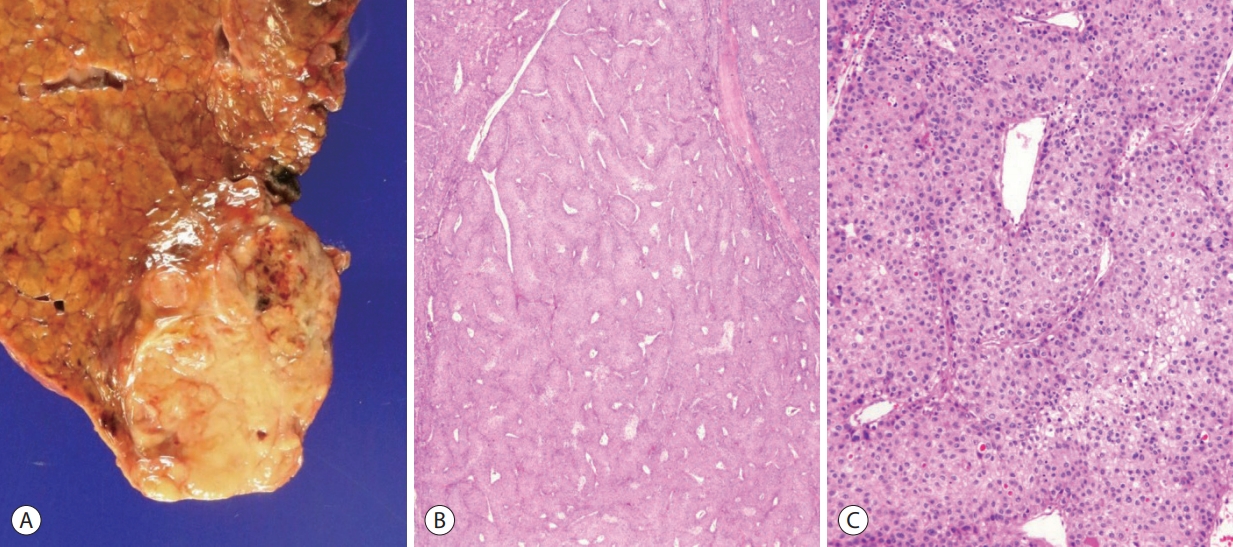
Hepatocellular carcinoma, macrotrabecular massive variant. (A) Gross feature of the tumor. (B) At low power magnification, thick tumor cell trabeculae are seen in more than 50% of the tumor (hematoxylin-eosin stain, ×12.5). (C) The tumor cell trabeculae are more than 10 cells thick (hematoxylin-eosin stain, ×100).
SCIRRHOUS VARIANT
Scirrhous HCC is characterized by dense intratumoral fibrous stroma, occupying at least 30-50% of the tumor (Fig. 5) [11,18-20]. The tumor cell nests are predominantly composed of mature hepatocyte-like cells and rimmed by smaller tumor cells that resemble hepatic stem/progenitor cells – the latter population frequently express stemness-related markers, such as CK19, CK7, and epithelial cell adhesion molecule [19,20]. This variant accounts for 4% of HCCs, and the dense fibrous stroma results in imaging findings that overlap with those of cholangiocarcinoma. TSC1/TSC2 mutations and transforming growth factor-beta signaling activation have been demonstrated in these tumors [11,20]. The clinical outcome of this variant is still controversial. However, scirrhous HCCs have been reported to demonstrate decreased survival compared to conventional HCCs, especially in larger tumors (≥5 cm), and aggressive pathological features (e.g. vascular invasion, infiltrative growth) have been more frequently seen in these tumors compared to conventional HCCs [20,21].
CHROMOPHOBE VARIANT
This is a rare (up to 3%) variant of HCC that is characterized by clear (chromophobic) cytoplasm of the tumor cells, and focal areas of striking nuclear atypia in a background of otherwise “bland-looking” cytology, and thus have been initially named “chromophobe HCC with abrupt anaplasia” [22]. From a molecular viewpoint, it is interesting that the majority of the reported cases demonstrated alternative lengthening of telomere phenotype by telomere fluorescence in situ hybridization (FISH) [22]. Little is known regarding the clinical correlates of this variant, and the prognosis so far seems to be similar to conventional HCCs.
FIBROLAMELLAR HCC (FIBROLAMELLAR CARCINOMA)
The fibrolamellar subtype of HCC had already been designated as a subtype of HCC in the previous WHO classification [6], and has unique clinical characteristics, including the age distribution (children and young adults) and the absence of underlying chronic liver disease [1,23]. It is a rare subtype that accounts for up to 1% of HCCs and occurs more frequently in Western countries [1]. Histologically, it is characterized by parallel arrays of large eosinophilic tumour cells with large vesicular nuclei and prominent nucleoli, and separated by dense fibrous septa. Due to the abundance of fibrous intratumoral stroma, it may appear similar to scirrhous HCC; however, the fibrous stroma of scirrhous HCC has been recently shown to contain more abundant cancer-associated fibroblasts and tumor-associated macrophages, and stemness-related marker expression is more frequently seen in scirrhous HCCs.24 Recently, DNAJB1-PRKACA gene fusion has been demonstrated in fibrolamellar HCC, and hence FISH for PRKACA gene rearrangement has been proposed to be a good diagnostic tool for this tumor [25,26]. The prognosis of fibrolamellar HCC has been shown to be better than for conventional HCCs that arise in cirrhotic livers, but similar to HCCs in non-cirrhotic livers.27 In addition, conventional HCCs with features of fibrolamellar HCC in parts of the tumor have been shown to have similar prognosis with conventional HCCs, suggesting that a strict criterion (fibrolamellar features in the entire tumor) is required for a diagnosis of fibrolamellar HCC [28].
NEUTROPHIL-RICH VARIANT
This is a very rare variant of HCCs (less than 1%) that is characterized histologically by diffuse neutrophilic infiltration within the HCC, and clinically by elevated leukocyte counts, C-reactive protein and IL-6, and a poor prognosis compared to conventional HCCs. The tumor cells may have focal sarcomatoid appearance, and granulocyte colony-stimulating factor production by the tumor cells have been reported [29].
LYMPHOCYTE-RICH VARIANT
The lymphocyte-rich variant of HCC has been shown to account for <1% of HCCs, and demonstrates massive intratumoral lymphocytic infiltration (Fig. 6). It has also been referred to as lymphoepithelioma-like HCCs [29,30]. These tumors have been associated with a favorable clinical outcome and also more frequent programmed death-ligand 1 expression [31-33]. The molecular features of this subtype have not been characterized yet; however, an association with Epstein-Barr virus infection has not been demonstrated, unlike similar tumors of some other organs such as the stomach [32]. Interestingly, a minority of HCCs also demonstrates lymphoid aggregates or follicles within the tumor, and the presence of these tertiary lymphoid structures has been associated with a favorable prognosis [34].
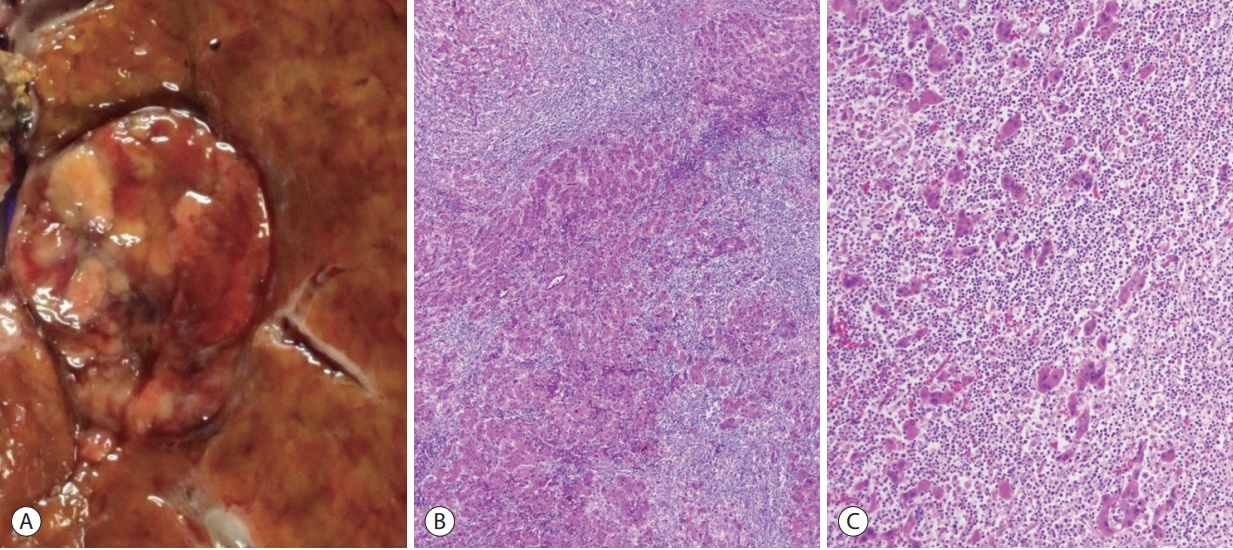
Hepatocellular carcinoma, lymphocyte-rich variant. (A) Gross appearance of the tumor. (B) At low power magnification, the tumor is massively infiltrated by lymphocytes (hematoxylin-eosin stain, ×40). (C) The tumor cell trabeculae or nests are separated by dense lymphoid cell infiltrates (hematoxylin-eosin stain, ×100).
CONCLUSION
Following the accumulation of clinicopathological studies on various histopathological forms of HCC and the increasing number of molecular studies addressing the heterogeneity of HCC, there have been efforts to connect the histomorphological and molecular features of HCCs, and such links have resulted in the currently proposed HCC variants of the recent WHO Classification of Digestive System Tumors [1]. With the recent morpho-molecular correlations of HCCs and the identification of various histopathological variants of HCC, most of which are based on their molecular features, we can now not only better understand the pathogenesis of HCC, but also use this knowledge to predict patient outcome and to facilitate the development of targeted therapy. More importantly, the identification of histopathological variants of HCCs suggests that histopathology still has an important role in HCC patient management.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2019R1A2C2010056, NRF-2016R1D1A1A09919042 to HK, NRF-2020R1A2B5B01001646, NRF-2017M3A9B6061512, and NRF-2016M3A9D5A01952416 to YNP).


