Articles
- Page Path
- HOME > J Liver Cancer > Volume 23(2); 2023 > Article
-
Review Article
Diagnosis of hepatocellular carcinoma using Sonazoid: a comprehensive review -
Woo Kyoung Jeong1,2

-
Journal of Liver Cancer 2023;23(2):272-283.
DOI: https://doi.org/10.17998/jlc.2023.08.25
Published online: September 19, 2023
1Department of Radiology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
2Center for Imaging Sciences, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
-
Corresponding author: Woo Kyoung Jeong, Department of Radiology, Samsung Medical Center, Sungkyunkwan University School of Medicine, 81 Irwon-ro, Gangnam-gu, Seoul 06351, Korea
Tel. +82-2-3410-1923, Fax. +82-2-3410-0049 E-mail: jeongwk@gmail.com
© 2023 The Korean Liver Cancer Association.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,211 Views
- 85 Downloads
- 1 Citation
- Abstract
- INTRODUCTION
- SONAZOID CEUS: TECHNICAL PERSPECTIVES
- HOW TO PERFORM SONAZOID CEUS
- STANDARDIZATION OF SONAZOID CEUS OBSERVATIONS: MODIFICATION OF THE LIVER IMAGING REPORTING AND DATA SYSTEM
- IMAGING DIAGNOSIS OF FOCAL HEPATIC LESIONS
- INTRODUCTION OF CURRENT GUIDELINES FOR HCC DIAGNOSIS USING SONAZOID PUBLISHED BY THE KOREAN SOCIETY OF RADIOLOGY AND KOREAN SOCIETY OF ABDOMINAL RADIOLOGY
- CONCLUSION
- Article information
- References
Abstract
- Sonazoid contrast-enhanced ultrasonography (CEUS) is a promising technique for the detection and diagnosis of focal liver lesions, particularly hepatocellular carcinoma (HCC). Recently, a collaborative effort between the Korean Society of Radiology and Korean Society of Abdominal Radiology resulted in the publication of guidelines for diagnosing HCC using Sonazoid CEUS. These guidelines propose specific criteria for identifying HCC based on the imaging characteristics observed during Sonazoid CEUS. The suggested diagnostic criteria include nonrim arterial phase hyperenhancement, and the presence of late and mild washout, or Kupffer phase washout under the premise that the early or marked washout should not occur during the portal venous phase. These criteria aim to improve the accuracy of HCC diagnosis using Sonazoid CEUS. This review offers a comprehensive overview of Sonazoid CEUS in the context of HCC diagnosis. It covers the fundamental principles of Sonazoid CEUS and its clinical applications, and introduces the recently published guidelines. By providing a summary of this emerging technique, this review contributes to a better understanding of the potential role of Sonazoid CEUS for diagnosing HCC.
- Hepatocellular carcinoma (HCC) is a prevalent and lethal cancer, and early detection and accurate diagnosis are crucial for improving patient outcomes.1-4 Various imaging modalities, including ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI) using hepatocyte-specific contrast agents, have been used for HCC diagnosis. Recently, contrast-enhanced ultrasound (CEUS) with perfluorobutane (SonazoidTM; GE Healthcare, Milwaukee, WI, USA) has emerged as a promising tool for HCC diagnosis.5 Perfluorobutane microbubbles have unique properties, allowing them to be selectively engulfed by intrasinusoidal macrophages (Kupffer cells); this leads to sustained enhancement of the hepatic parenchyma during CEUS examinations,6,7 and Kupffer phase washout of hepatic malignancies, thereby indicating the defect by enhancing the surrounding parenchyma.8-10 Additionally, this enables the real-time visualization of arterial phase hyperenhancement (APHE), which other imaging modalities cannot achieve. Sonazoid CEUS offers various advantages, such as real-time imaging, high temporal resolution, and safety without ionizing radiation or nephrotoxic agents. In Korea, Sonazoid is a contrast agent for CEUS approved by the Ministry of Food and Drug Safety in 2012, indicated for use as a medical imaging technique for the diagnosis of focal hepatic lesions, including HCC.11
- This review aims to provide an overview of Sonazoid CEUS for the diagnosis of HCC, including its principles, clinical applications, and introduction of current guidelines.
INTRODUCTION
- 1. Development of Sonazoid
- Ultrasound has long been used in medical imaging due to its noninvasive nature and lack of ionizing radiation; however, its ability to differentiate between various tissues and visualize blood flow is limited. In 1969, researchers investigated the use of agitated saline as the contrast agent for echocardiography,12 while the use of microbubble-based contrast agents was explored to enhance ultrasound imaging.13-15 Microbubbles are small, gas-filled spheres that can be injected into the bloodstream to improve the visibility of blood vessels and tissue perfusion. Early microbubble contrast agents were composed of air or nitrogen gas, but had limited stability and persistence in the circulation. To overcome these limitations, scientists considered perfluorocarbon gases, which were identified as promising candidates for CEUS due to their favorable physical properties, including a high solubility and stability.
- Perfluorobutane, a kind of perfluorocarbon gas, demonstrates excellent biological stability with a long intravascular half-life and minimal metabolism in the body.16 It has favorable pharmacokinetics characterized by slow elimination from the bloodstream and low systemic absorption, contributing to its safety profile as a contrast agent for ultrasound imaging.17,18 Several studies have demonstrated its minimal accumulation in organs and tissues; additionally, it is exhaled via the lungs, supporting its overall safety.6,7,19,20 Despite the lack of research regarding the frequency of adverse events after Sonazoid administration, there are potential side effects, including allergic reactions, cardiopulmonary distress, and rare instances of anaphylaxis, as with other ultrasound contrast agents.21 Regarding contraindications, Sonazoid is contraindicated in patients with known hypersensitivity to perfluorocarbon-based contrast agents or severe cardiopulmonary impairment, and in cases where left-to-right shunting is suspected, as it may exacerbate shunting and lead to systemic embolization.
- 2. Characteristics of Sonazoid
- Sonazoid comprises microbubbles filled with perfluorobutane gas surrounded by a monomolecular membrane of hydrogenated egg phosphatidyl serine embedded in an amorphous sucrose structure.22 The average size of the microbubbles is 2.6 µm, which too large to pass into the perisinusoidal tissue. Thus, it is a pure blood pool agent without leakage into the extracellular space, with imaging characteristics different from those of CT and MRI whose contrast agents can leak and disperse into extracellular spaces during contrast enhancement. Sonazoid can therefore provide enhanced visualization of blood flow in hepatic tumors without extravascular enhancement.
- The Kupffer phase in Sonazoid CEUS refers to the postvascular phase when the contrast agent is taken up by Kupffer cells, specialized cells responsible for phagocytosis in the hepatic sinusoid. Kupffer phase washout, which is the absence of Kupffer cell uptake, usually appears in hepatic malignancies and is a crucial finding for diagnosing and characterizing liver lesions. Moreover, several studies have reported that CEUS examinations incorporating the Kupffer phase help improve the diagnostic performance, especially sensitivity, for HCC.23-25
SONAZOID CEUS: TECHNICAL PERSPECTIVES
- Sonazoid CEUS is a real-time ultrasonographic examination performed after injecting a contrast agent. Prior to injection, a mixture of Sonazoid powder and distilled water is prepared in a package. The amount of contrast agent injected is calculated based on the patient’s body weight, with a dosage of 0.015 mL/kg. After bolus injection of the contrast agent, a flush of 10 mL normal saline is administered.
- In addition to contrast agent preparation, proper settings for the ultrasonographic machine are crucial. This is because the microbubbles employed in CEUS studies are delicate and cannot withstand the ultrasound power used in conventional ultrasound imaging; therefore, the mechanical index of the machine is adjusted to a lower range of 0.2-0.3, and it is recommended to fine-tune the settings according to the manufacturer’s instructions.
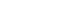
- Sonazoid CEUS consists of different phases: the arterial phase, early portal venous phase (up to a 1-minute delay), late portal venous phase (up to a 2-minute delay), and Kupffer phase (Fig. 1).5 The arterial phase begins when microbubbles are visualized in the hepatic artery, and typically lasts for 20-35 seconds. Following the arterial phase, the portal venous phase immediately begins and continues for 2 minutes after injection. It is important to distinguish between the early and late portal venous phases as the timing of tumor washout is critical for diagnosing HCC. The Kupffer phase starts approximately 10 minutes after injection, although the World Federation for Ultrasound in Medicine and Biology guideline suggests an 8-minute starting point.21,26
HOW TO PERFORM SONAZOID CEUS
- Over the past two decades, various medical societies have proposed imaging-based criteria and guidelines for diagnosing HCC.1,4 These systems originated from Europe, Asia, and North America; thus, inconsistencies led to regional differences and hindered the global adoption of a single guideline. Sonazoid is not universally used for the diagnosis of HCC; however, Asian guidelines, such as the Korean Liver Cancer Association, Asian Pacific Association for the Study of the Liver, and Japan Society of Hepatology guidelines, recommend that Sonazoid CEUS be used as a second-line imaging study in case of indeterminate cases on MRI and/or CT. The Liver Imaging Reporting and Data System (LI-RADS) aims to unify HCC imaging and diagnosis worldwide.27,28 A unified system would improve clinical care, promote the creation of registries, enable multicentric and international studies with standardized reporting criteria, and facilitate meta-analyses of published literature; however, Sonazoid CEUS is not yet included in the latest system.
- Nevertheless, the establishment of the LI-RADS and its comprehensive lexicon of precisely defined radiologic terms has addressed the challenges posed by ambiguous and inconsistent terminology regarding the imaging literature on HCC.29 This standardization has improved communication and clarity in clinical care, as well as facilitated research and education in the field of HCC imaging. Observations within the LI-RADS encompass a wide range of possibilities, spanning from benign to malignant, and these lesions can originate from hepatocellular or nonhepatocellular sources. While HCC is the most common primary liver malignancy in the LI-RADS target population, there are other primary malignancies that may occur, such as intrahepatic cholangiocarcinoma (ICC) and combined hepatocellular cholangiocarcinoma. These hepatic lesions have specific imaging characteristics categorized into major and ancillary features of CT or MRI, and combinations of these features (such as APHE and washout in the portal venous phase) can indicate the right diagnosis in many cases.
- The CEUS LI-RADS algorithm shares similarities with the CT/MRI LI-RADS algorithm, with some modifications; one key difference is the characterization of washout. Unlike CT and MRI contrast agents, the microbubbles used in CEUS remain in the blood pool and do not leak into the tumor or surrounding tissue. The distribution of microbubbles in the postarterial phase images reflects the relative blood volume. This distinction allows CEUS LI-RADS to assess degree and onset time of washout, such as late or mild washout, which can differentiate between HCC and other non-HCC malignancies. Another important distinction is the absence of vascular pseudolesions, such as arterioportal shunt.30 Any observation showing APHE in CEUS can be considered a true arterialized nodule. This distinction is especially important in cirrhosis, where most nodules with arterial enhancement are likely to be premalignant or malignant and should be categorized as LR-4 or higher on CEUS LI-RADS.31
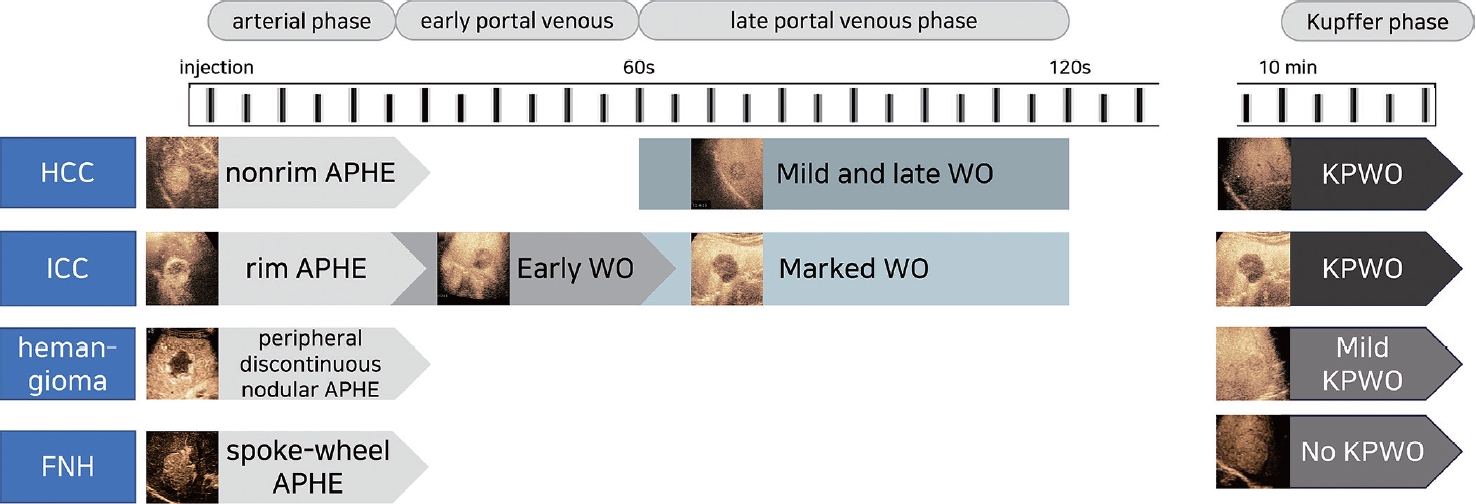
- In the CEUS examination, there are four hyperenhancement and four washout categories: nonrim APHE, rim APHE, peripheral discontinuous nodular APHE, and spoke-wheel APHE in the hyperenhancement category (Fig. 2); and early washout, late washout, mild washout, and marked washout (punched-out) in the washout category. Nonrim APHE refers to the presence of arterial phase enhancement within a hepatic lesion that lacks a rim-like pattern. It can be caused by factors such as irregular vascularity or arterioportal shunts. By contrast, rim APHE refers to the presence of a peripheral or rim-like pattern of enhancement around a hepatic lesion during the arterial phase. This pattern suggests a rapid and intense uptake of microbubbles by the lesion’s peripheral vessels. Peripheral discontinuous nodular APHE refers to the presence of nodular enhancement in a peripheral or rim-like pattern around a liver lesion with interruptions or gaps in the enhancement, and it is usually seen in hepatic hemangiomas. Last, spoke-wheel APHE refers to a unique pattern of enhancement resembling the spokes of a wheel radiating from the center of a liver lesion during the arterial phase; this pattern is typically associated with focal nodular hyperplasia (FNH).
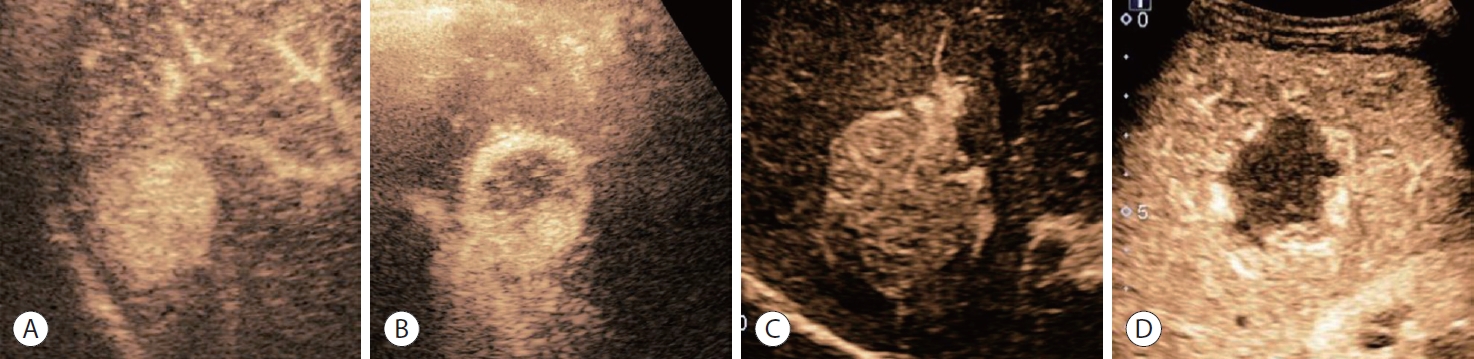
- Washout can be classified by the degree and onset time of washout (Fig. 3). The degree of washout indicates the extent to which the lesion’s enhancement decreases compared with the arterial phase. A mild washout indicates a partial reduction in enhancement, with residual enhancement still present. Conversely, a marked washout indicates a near-complete loss of enhancement, with minimal or no residual enhancement observed. The onset time of washout refers to the timing at which the decrease in enhancement becomes evident, with early onset indicating a rapid decline in enhancement (within 60 seconds), and late onset indicating a gradual decrease over a longer period (>60 seconds). Portal vein tumor thrombosis is frequently shown in infiltrative HCC, and CEUS shows early-enhancing soft-tissue in the portal vein, rather than the flow of microbubbles in the vessel.30 Mosaic and nodule in nodule architecture may be findings that favor HCC, and are considered ancillary features favoring malignancy in CEUS LI-RADS (Fig. 4).32
STANDARDIZATION OF SONAZOID CEUS OBSERVATIONS: MODIFICATION OF THE LIVER IMAGING REPORTING AND DATA SYSTEM
- 1. Hepatocellular carcinoma
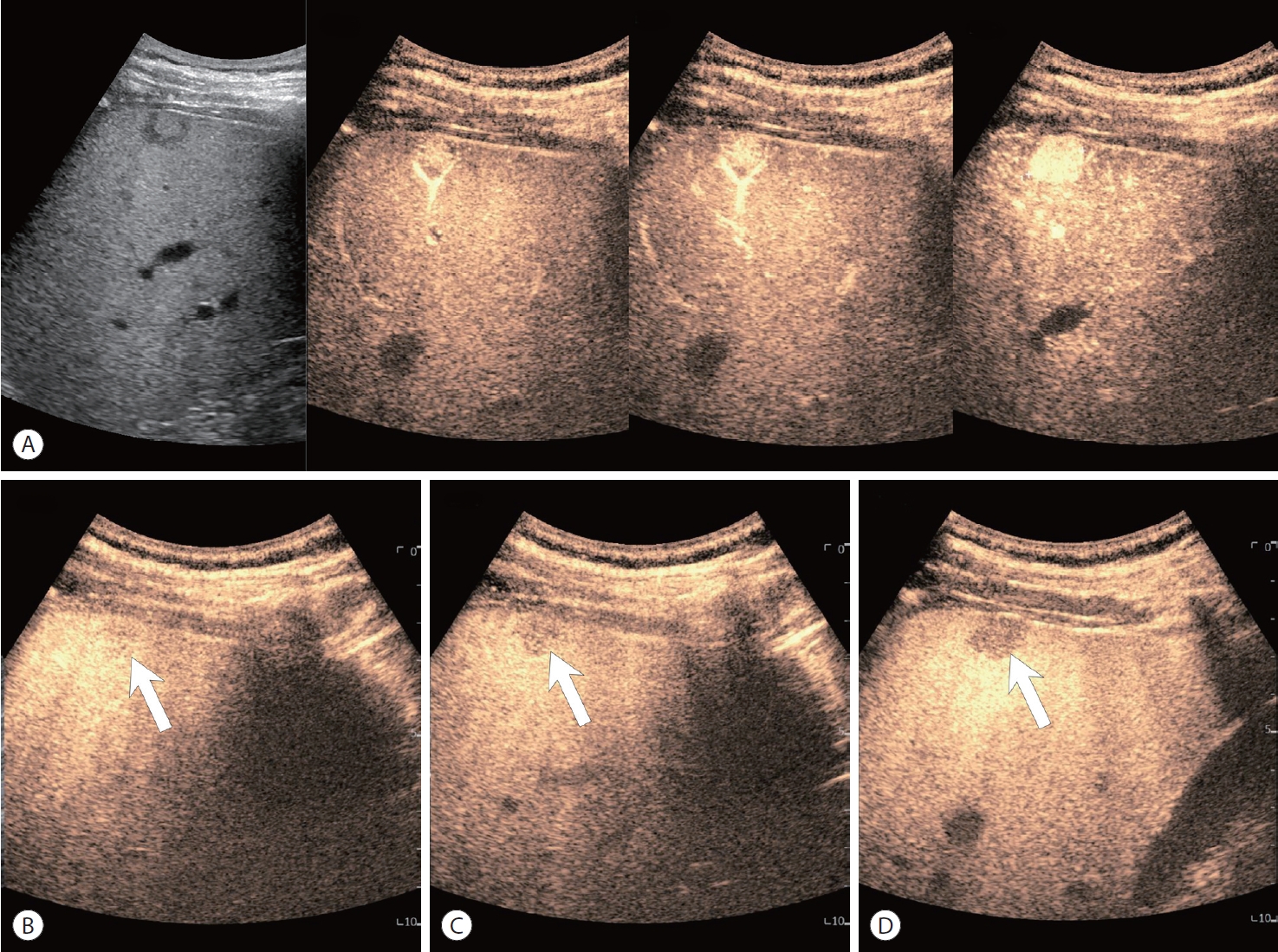
- CEUS can also be used for the diagnosis of HCC, and shows different imaging characteristics to CT or MRI due to the presence of microbubbles that are confined to the vascular space or engulfed by Kupffer cells. Typically, CEUS shows a characteristic pattern of nonrim APHE and ‘late and mild’ washout starting around 1 minute after contrast injection in HCC (Fig. 5). CEUS can also help differentiate simple thrombus from tumorous thrombus in the portal vein. According to CEUS LI-RADS, where the presence of nonrim arterial hyperenhancement and delayed mild washout corresponds with a diagnosis of LR-5, peripheral enhancement or early and marked washout indicates LR-M, suggesting nonHCC malignant liver tumors. It should be noted that CEUS with Sonazoid contrast agent cannot currently support CEUS LI-RADS, and is limited to blood pool agents like Sonovue.
- 2. Intrahepatic cholangiocarcinoma
- ICC is the second most common primary malignant liver tumor, and generally has a poorer prognosis than HCC. It often presents with intrahepatic bile duct dilation; however, smaller ICCs can appear as nodules showing unclear ductal expansion and arterial enhancement, requiring differentiation from HCC. Contrast-enhanced ultrasound findings of ICC show rim APHE, and early and marked washout occurring relatively soon after arterial enhancement (Fig. 6), distinguishing it from the delayed washout pattern seen in HCC. Additionally, while peripheral enhancement may be observed during the arterial phase, it is not a specific finding and can also be seen in HCC.
- 3. Dysplastic nodule
- There are many regenerative or dysplastic nodules in the cirrhotic liver tissue, resulting in a high rate of false-positive diagnoses using B-mode ultrasound. There is little evidence of CEUS findings of cirrhotic nodules; however, Kupffer cells are usually preserved in these nodules, which makes a clear case for the benefit of Sonazoid CEUS compared with Bmode ultrasound for reducing false referral.33
- 4. Hemangioma
- Hepatic hemangiomas are particularly common benign liver tumors that exhibit noncontinuous nodular enhancement along the periphery on CT or MRI. Over time, the size of the enhancing nodules increases, and centripetal enhancement (progressing from the periphery toward the center of the lesion) can be observed. However, caution should be exercised, as some hepatic hemangiomas may show a ring-shaped enhancement pattern, which can mimic nonspecific HCC or ICC.34,35 While most hepatic hemangiomas maintain enhancement without washout during the portal and delayed phases, the use of Sonazoid may result in mild hypoenhancement on Kupffer imaging (Fig. 7), although the internal echogenicity of the lesion tends to be slightly higher compared with typical malignant lesions.24
- 5. FNH
- FNH is a benign condition characterized by localized nodular overgrowth, commonly observed in females, and occasionally in males. Imaging features of FNH include a central scar and a spoke-wheel pattern of vessels within the lesion, which can be visualized on contrast-enhanced ultrasound. Sonazoid contrast-enhanced ultrasound, along with the additional use of Kupffer imaging, can be helpful for diagnosing FNH. Since FNH comprises normal liver cells and retains normal Kupffer cells within the hepatic veins, there is minimal washout of contrast agent, and only partial washout may be seen in the central scar.36,37
IMAGING DIAGNOSIS OF FOCAL HEPATIC LESIONS
- In 2023, the Korean Society of Radiology and Korean Society of Abdominal Radiology published guidelines for diagnosing hepatocellular carcinoma using Sonazoid contrast-enhanced ultrasonography.5 Key questions and recommendations with explanations follow.
- 1. Is it appropriate for nonrim APHE and late (≥60 seconds) and mild washout to be major imaging features of HCC in Sonazoid CEUS?
- During hepatocarcinogenesis, the density of unpaired arteries increases, leading to more enhancement in the arterial phase compared with the liver. This nonrim APHE is a key imaging feature for diagnosing HCC in at-risk patients.2,38 However, certain types of APHE, such as rim, spoke-wheel, centrifugal, and peripheral discontinuous nodular APHE, should not be considered major findings for HCC diagnosis on Sonazoid CEUS due to their association with other conditions. Diffuse, nonrim APHE is a crucial finding in HCC on Sonazoid CEUS. Studies have shown that nonrim APHE is more frequently observed in HCC than in non-HCC malignancies or benign lesions.25 Additionally, washout appearance, defined as a temporal reduction in enhancement, is another major imaging feature for HCC diagnosis in at-risk patients. Late (≥60 seconds) and mild washout on Sonazoid CEUS were found to be important factors for differentiating HCC from other malignancies.23,25,26,39,40
- 2. Can Kupffer phase washout be used as a major feature of HCC diagnosis in Sonazoid CEUS?
- Kupffer phase washout (characterized by hypoenhancement, compared with the liver in the Kupffer phase of Sonazoid CEUS) reflects a decrease in Kupffer cells, and can be considered a major imaging feature for diagnosing HCC in at-risk patients.24,40,41 It has demonstrated improved sensitivity for detecting HCC compared with late and mild washout. This washout pattern in the Kupffer phase is more common in early HCCs, nodule-in-nodule type HCCs, and well-differentiated HCCs;42,43 however, it is not specific to HCC and may reduce specificity. Therefore, exceptions, such as excluding early or marked washout during the vascular phase, should be considered when assessing Kupffer phase washout. Studies have shown that metastases, ICC, and combined hepatocellular cholangiocarcinoma typically exhibit rim APHE, early washout, or marked washout, distinguishing them from HCC.28 When comparing diagnostic criteria, the combination of nonrim APHE and Kupffer phase washout provides higher sensitivity and accuracy for HCC diagnosis without sacrificing specificity.
- 3. What are the appropriate criteria for diagnosing HCC using Sonazoid CEUS in at-risk patients?
- Several studies have examined the use of Sonazoid CEUS for diagnosing HCC;24,25,40,44-46 however, these studies have limitations, such as being retrospective and lacking clear diagnostic criteria. The CEUS LI-RADS criteria, originally developed for blood-pool agents,28 may be applicable to Sonazoid CEUS with modifications. Retrospective studies have shown that incorporating Kupffer phase washout in the modified CEUS LI-RADS criteria improves sensitivity without a significant loss of specificity.24,25 Based on these findings, the proposed guidelines suggest diagnosing HCC in at-risk patients when nonrim APHE is present with late and mild washout, or Kupffer phase washout.
- 4. Can Sonazoid CEUS be used to characterize inconclusive nodules detected in CT or MRI in patients at high risk of HCC?
- Sonazoid CEUS is recommended as a second-line imaging modality for evaluating nodules that are inconclusive on contrast-enhanced CT or MRI.2,3 It offers several advantages, such as excellent temporal resolution and real-time monitoring of the liver during the early vascular phase, allowing for the detection of arterial hypervascularity that may be missed on CT or MRI. Previous studies have shown that Sonazoid CEUS identified arterial hypervascularity in a significant percentage of lesions that did not exhibit hypervascularity on CT or MRI.10,47 Additionally, Sonazoid CEUS provides the ability to acquire Kupffer phase images, allowing for further characterization of inconclusive nodules. From a safety perspective, Sonazoid CEUS is considered safe and free from radiation hazards.
- 5. Can Sonazoid CEUS differentiate HCC from non-HCC malignancies?
- Sonazoid CEUS is helpful for distinguishing HCC from ICC and metastases.23,24,48-50 ICC on Sonazoid CEUS exhibits early or marked washout, while metastases from HCC show rim APHE and hypoenhancement in the later phases. However, HCC, ICC, and metastases may present with overlapping features, making a clear CEUS-based diagnosis challenging.
- 6. Can Sonazoid CEUS be used as a surveillance tool for HCC in high-risk patients?
- Sonazoid CEUS may be a useful tool for the surveillance of HCC in high-risk patients, particularly those with cirrhosis and multiple hepatic nodules. It provides a stable and extended Kupffer phase, allowing for the detection of HCCs presenting as hypoechoic lesions with the background of enhanced parenchyma. Several studies have demonstrated the superior sensitivity of Sonazoid CEUS for detecting small HCCs compared with B-mode ultrasound alone.9,51,52 Adding Sonazoid CEUS to ultrasound surveillance was shown to increase the detection rate of early-stage HCC and reduce false referrals. Cost-effectiveness analyses targeting hepatitis C viral infections in Japan suggest that Sonazoid CEUS is a cost-effective surveillance modality.53 Although there are some prerequisites, such as regional deviations, medical costs, and kinds of dominant liver diseases, Sonazoid CEUS holds promise as a valuable surveillance tool for the early detection of HCC in high-risk patients if the exam cost is reasonably set.
- 7. Is Sonazoid CEUS helpful for guiding local ablation therapy for HCC?
- Sonazoid CEUS has advantages over B-mode ultrasound in guiding local ablation therapy for HCC due to its improved lesion detectability and increased sensitivity. Kupffer phase imaging of Sonazoid CEUS allows for better visualization of small HCCs, which can be challenging with B-mode ultrasound.40 Studies have shown that Sonazoid CEUS-guided radiofrequency ablation detects more HCC nodules and achieves higher detection rates than B-mode ultrasound alone.54-56 Additionally, Sonazoid CEUS guidance reduces the number of treatment sessions required and improves therapeutic outcomes by providing sustained local tumor control. Ultrasound fusion imaging with Sonazoid CEUS further enhances the accuracy and feasibility of local ablation therapy for HCC.56
- 8. Is it appropriate to use Sonazoid CEUS to assess the treatment response of HCC in patients who underwent transarterial chemoembolization or radiofrequency ablation?
- Sonazoid CEUS shows promise for evaluating treatment response for HCC after locoregional therapies and systemic treatments.57-60 However, more extensive studies are needed for stronger recommendations, as current evidence is limited. Sonazoid CEUS can be a supplementary option when evaluating treatment response for HCC, especially when few index tumors are observed.
INTRODUCTION OF CURRENT GUIDELINES FOR HCC DIAGNOSIS USING SONAZOID PUBLISHED BY THE KOREAN SOCIETY OF RADIOLOGY AND KOREAN SOCIETY OF ABDOMINAL RADIOLOGY
- In conclusion, Sonazoid CEUS is a valuable imaging technique that enhances the visualization of blood flow, tissue perfusion in the liver, and Kupffer cell uptake imaging. Its real-time capabilities and non-invasive nature make it a useful tool for diagnosing HCC, as well as monitoring different pathologies, ultimately improving patient care and treatment outcomes.
CONCLUSION
-
Conflict of Interest
The author has no conflicts of interest to disclose.
-
Ethics Statement
This review article is fully based on articles which have already been published and did not involve additional patient participants. Therefore, IRB approval is not necessary.
-
Funding Statement
None.
-
Data Availability
Not applicable.
-
Author Contribution
Conceptualization: WKJ
Project administration: WKJ
Visualization: WKJ
Writing–original draft: WKJ
Writing–review & editing: WKJ
Article information




- 1. European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2018;69:182−236.ArticlePubMed
- 2. Korean Liver Cancer Association (KLCA), National Cancer Center (NCC) Korea. 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. Korean J Radiol 2022;23:1126−1240.ArticlePubMedPMCPDF
- 3. Korean Liver Cancer Association (KLCA), National Cancer Center (NCC) Korea. 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. J Liver Cancer 2023;23:1−120.ArticlePubMedPMCPDF
- 4. Singal AG, Llovet JM, Yarchoan M, Mehta N, Heimbach JK, Dawson LA, et al. AASLD practice guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology 2023;May 22 doi: 10.1097/HEP.0000000000000466. [Epub ahead of print].Article
- 5. Jeong WK, Kang HJ, Choi SH, Park MS, Yu MH, Kim B, et al. Diagnosing hepatocellular carcinoma using sonazoid contrast-enhanced ultrasonography: 2023 guidelines from the Korean Society of Radiology and the Korean Society of Abdominal Radiology. Korean J Radiol 2023;24:482−497.ArticlePubMedPMCPDF
- 6. Watanabe R, Matsumura M, Munemasa T, Fujimaki M, Suematsu M. Mechanism of hepatic parenchyma-specific contrast of microbubble-based contrast agent for ultrasonography: microscopic studies in rat liver. Invest Radiol 2007;42:643−651.PubMed
- 7. Yanagisawa K, Moriyasu F, Miyahara T, Yuki M, Iijima H. Phagocytosis of ultrasound contrast agent microbubbles by Kupffer cells. Ultrasound Med Biol 2007;33:318−325.ArticlePubMed
- 8. Moriyasu F, Itoh K. Efficacy of perflubutane microbubble-enhanced ultrasound in the characterization and detection of focal liver lesions: phase 3 multicenter clinical trial. AJR Am J Roentgenol 2009;193:86−95.ArticlePubMed
- 9. Kudo M, Hatanaka K, Kumada T, Toyoda H, Tada T. Doublecontrast ultrasound: a novel surveillance tool for hepatocellular carcinoma. Am J Gastroenterol 2011;106:368−370.ArticlePDF
- 10. Mandai M, Koda M, Matono T, Nagahara T, Sugihara T, Ueki M, et al. Assessment of hepatocellular carcinoma by contrast-enhanced ultrasound with perfluorobutane microbubbles: comparison with dynamic CT. Br J Radiol 2011;84:499−507.ArticlePubMedPMC
- 11. Lee JY, Minami Y, Choi BI, Lee WJ, Chou YH, Jeong WK, et al. The AFSUMB consensus statements and recommendations for the clinical practice of contrast-enhanced ultrasound using Sonazoid. Ultrasonography 2020;39:191−220.ArticlePubMedPMCPDF
- 12. Gramiak R, Shah PM, Kramer DH. Ultrasound cardiography: contrast studies in anatomy and function. Radiology 1969;92:939−948.ArticlePubMed
- 13. Calliada F, Campani R, Bottinelli O, Bozzini A, Sommaruga MG. Ultrasound contrast agents: basic principles. Eur J Radiol 1998;27 Suppl 2:S157−S160.PubMed
- 14. Lee H, Kim H, Han H, Lee M, Lee S, Yoo H, et al. Microbubbles used for contrast enhanced ultrasound and theragnosis: a review of principles to applications. Biomed Eng Lett 2017;7:59−69.ArticlePubMedPMCPDF
- 15. Chung YE, Kim KW. Contrast-enhanced ultrasonography: advance and current status in abdominal imaging. Ultrasonography 2015;34:3−18.ArticlePubMedPMC
- 16. Sontum PC, Ostensen J, Dyrstad K, Hoff L. Acoustic properties of NC100100 and their relation with the microbubble size distribution. Invest Radiol 1999;34:268−275.ArticlePubMed
- 17. Landmark KE, Johansen PW, Johnson JA, Johansen B, Uran S, Skotland T. Pharmacokinetics of perfluorobutane following intravenous bolus injection and continuous infusion of sonazoid in healthy volunteers and in patients with reduced pulmonary diffusing capacity. Ultrasound Med Biol 2008;34:494−501.ArticlePubMed
- 18. Li P, Hoppmann S, Du P, Li H, Evans PM, Moestue SA, et al. Pharmacokinetics of perfluorobutane after intra-venous bolus injection of Sonazoid in healthy Chinese volunteers. Ultrasound Med Biol 2017;43:1031−1039.ArticlePubMed
- 19. Uran S, Landmark K, Normann PT, Hals PA, Toft KG, Skotland T. A respiration-metabolism chamber system and a GC-MS method developed for studying exhalation of perfluorobutane in rats after intravenous injection of the ultrasound contrast agent Sonazoid. J Pharm Biomed Anal 2005;39:746−751.ArticlePubMed
- 20. Toft KG, Hustvedt SO, Hals PA, Oulie I, Uran S, Landmark K, et al. Disposition of perfluorobutane in rats after intravenous injection of Sonazoid. Ultrasound Med Biol 2006;32:107−114.ArticlePubMed
- 21. Dietrich CF, Nolsøe CP, Barr RG, Berzigotti A, Burns PN, Cantisani V, et al. Guidelines and good clinical practice recommendations for contrast-enhanced ultrasound (CEUS) in the liver-update 2020 WFUMB in cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultrasound Med Biol 2020;46:2579−2604.ArticlePubMed
- 22. Sontum PC. Physicochemical characteristics of Sonazoid, a new contrast agent for ultrasound imaging. Ultrasound Med Biol 2008;34:824−833.ArticlePubMed
- 23. Kang HJ, Lee JM, Yoon JH, Lee K, Kim H, Han JK. Contrastenhanced US with sulfur hexafluoride and perfluorobutane for the diagnosis of hepatocellular carcinoma in individuals with high risk. Radiology 2020;297:108−116.ArticlePubMed
- 24. Sugimoto K, Kakegawa T, Takahashi H, Tomita Y, Abe M, Yoshimasu Y, et al. Usefulness of modified CEUS LI-RADS for the diagnosis of hepatocellular carcinoma using Sonazoid. Diagnostics (Basel) 2020;10:828. ArticlePubMedPMC
- 25. Hwang JA, Jeong WK, Min JH, Kim YY, Heo NH, Lim HK. Sonazoidenhanced ultrasonography: comparison with CT/MRI liver imaging reporting and data system in patients with suspected hepatocellular carcinoma. Ultrasonography 2021;40:486−498.ArticlePubMedPMCPDF
- 26. Kang HJ, Kim JH, Yoo J, Han JK. Diagnostic criteria of perfluorobutane-enhanced ultrasonography for diagnosing hepatocellular carcinoma in high-risk individuals: how is late washout determined? Ultrasonography 2022;41:530−542.ArticlePubMedPMCPDF
- 27. Tang A, Bashir MR, Corwin MT, Cruite I, Dietrich CF, Do RKG, et al. Evidence supporting LI-RADS major features for CT- and MR imaging-based diagnosis of hepatocellular carcinoma: a systematic review. Radiology 2018;286:29−48.ArticlePubMedPMC
- 28. Elsayes KM, Kielar AZ, Chernyak V, Morshid A, Furlan A, Masch WR, et al. LI-RADS: a conceptual and historical review from its beginning to its recent integration into AASLD clinical practice guidance. J Hepatocell Carcinoma 2019;6:49−69.PubMedPMC
- 29. Cunha GM, Fowler KJ, Roudenko A, Taouli B, Fung AW, Elsayes KM, et al. How to use LI-RADS to report liver CT and MRI observations. Radiographics 2021;41:1352−1367.ArticlePubMedPMC
- 30. Kim TK, Noh SY, Wilson SR, Kono Y, Piscaglia F, Jang HJ, et al. Contrast-enhanced ultrasound (CEUS) liver imaging reporting and data system (LI-RADS) 2017 - a review of important differences compared to the CT/MRI system. Clin Mol Hepatol 2017;23:280−289.ArticlePubMedPMCPDF
- 31. Jo PC, Jang HJ, Burns PN, Burak KW, Kim TK, Wilson SR. Integration of contrast-enhanced US into a multimodality approach to imaging of nodules in a cirrhotic liver: how i do it. Radiology 2017;282:317−331.ArticlePubMed
- 32. Bartolotta TV, Terranova MC, Gagliardo C, Taibbi A. CEUS LIRADS: a pictorial review. Insights Imaging 2020;11:9. ArticlePubMedPMCPDF
- 33. Ohama H, Imai Y, Nakashima O, Kogita S, Takamura M, Hori M, et al. Images of Sonazoid-enhanced ultrasonography in multistep hepatocarcinogenesis: comparison with Gd-EOB-DTPA-enhanced MRI. J Gastroenterol 2014;49:1081−1093.ArticlePubMedPDF
- 34. Kim TK, Lee E, Jang HJ. Imaging findings of mimickers of hepatocellular carcinoma. Clin Mol Hepatol 2015;21:326−343.ArticlePubMedPMCPDF
- 35. Kim TK, Choi BI, Han JK, Hong HS, Park SH, Moon SG. Hepatic tumors: contrast agent-enhancement patterns with pulse-inversion harmonic US. Radiology 2000;216:411−417.ArticlePubMed
- 36. Lee J, Jeong WK, Lim HK, Kim AY. Focal nodular hyperplasia of the liver: contrast-enhanced ultrasonographic features with Sonazoid. J Ultrasound Med 2018;37:1473−1480.ArticlePubMedPDF
- 37. Kang TW, Jeong WK, Kim YY, Min JH, Kim YK, Kim SH, et al. Comparison of super-resolution US and contrast material-enhanced US in detection of the spoke wheel sign in patients with focal nodular hyperplasia. Radiology 2021;298:82−90.ArticlePubMed
- 38. Chernyak V, Fowler KJ, Kamaya A, Kielar AZ, Elsayes KM, Bashir MR, et al. Liver imaging reporting and data system (LI-RADS) version 2018: imaging of hepatocellular carcinoma in at-risk patients. Radiology 2018;289:816−830.ArticlePubMedPMC
- 39. Saito A, Yamamoto M, Katagiri S, Yamashita S, Nakano M, Morizane T. Early hemodynamics of hepatocellular carcinoma using contrast-enhanced ultrasound with Sonazoid: focus on the pure arterial and early portal phases. Glob Health Med 2020;2:319−327.ArticlePubMedPMC
- 40. Hwang JA, Jeong WK, Kang HJ, Lee ES, Park HJ, Lee JM. Perfluorobutane-enhanced ultrasonography with a Kupffer phase: improved diagnostic sensitivity for hepatocellular carcinoma. Eur Radiol 2022;32:8507−8517.ArticlePubMedPDF
- 41. Inoue T, Kudo M, Hatanaka K, Takahashi S, Kitai S, Ueda T, et al. Imaging of hepatocellular carcinoma: qualitative and quantitative analysis of postvascular phase contrast-enhanced ultrasonography with sonazoid. Comparison with superparamagnetic iron oxide magnetic resonance images. Oncology 2008;75 Suppl 1:48−54.ArticlePDF
- 42. Kudo M, Hatanaka K, Inoue T, Maekawa K. Depiction of portal supply in early hepatocellular carcinoma and dysplastic nodule: value of pure arterial ultrasound imaging in hepatocellular carcinoma. Oncology 2010;78 Suppl 1:60−67.ArticlePubMedPDF
- 43. Takahashi M, Maruyama H, Ishibashi H, Yoshikawa M, Yokosuka O. Contrast-enhanced ultrasound with perflubutane microbubble agent: evaluation of differentiation of hepatocellular carcinoma. AJR Am J Roentgenol 2011;196:W123−W131.ArticlePubMed
- 44. Mita K, Kim SR, Kudo M, Imoto S, Nakajima T, Ando K, et al. Diagnostic sensitivity of imaging modalities for hepatocellular carcinoma smaller than 2 cm. World J Gastroenterol 2010;16:4187−4192.ArticlePubMedPMC
- 45. Goto E, Masuzaki R, Tateishi R, Kondo Y, Imamura J, Goto T, et al. Value of post-vascular phase (Kupffer imaging) by contrastenhanced ultrasonography using Sonazoid in the detection of hepatocellular carcinoma. J Gastroenterol 2012;47:477−485.ArticlePubMedPDF
- 46. Hsiao CY, Chen PD, Huang KW. A prospective assessment of the diagnostic value of contrast-enhanced ultrasound, dynamic computed tomography and magnetic resonance imaging for patients with small liver tumors. J Clin Med 2019;8:1353. ArticlePubMedPMC
- 47. Wang F, Numata K, Chuma M, Miwa H, Moriya S, Ogushi K, et al. A study on the inconsistency of arterial phase hypervascularity detection between contrast-enhanced ultrasound using sonazoid and gadolinium-ethoxybenzyl-diethylenetriamine penta-acetic acid magnetic resonance imaging of hepatocellular carcinoma lesions. J Med Ultrason (2001) 2021;48:215−224.ArticlePubMedPDF
- 48. Hatanaka K, Kudo M, Minami Y, Maekawa K. Sonazoid-enhanced ultrasonography for diagnosis of hepatic malignancies: comparison with contrast-enhanced CT. Oncology 2008;75 Suppl 1:42−47.ArticlePubMedPDF
- 49. Hatanaka K, Kudo M, Minami Y, Ueda T, Tatsumi C, Kitai S, et al. Differential diagnosis of hepatic tumors: value of contrast-enhanced harmonic sonography using the newly developed contrast agent, Sonazoid. Intervirology 2008;51 Suppl 1:61−69.ArticlePubMedPDF
- 50. Luo W, Numata K, Kondo M, Morimoto M, Sugimori K, Hirasawa K, et al. Sonazoid-enhanced ultrasonography for evaluation of the enhancement patterns of focal liver tumors in the late phase by intermittent imaging with a high mechanical index. J Ultrasound Med 2009;28:439−448.ArticlePubMedPDF
- 51. Kudo M, Ueshima K, Osaki Y, Hirooka M, Imai Y, Aso K, et al. Bmode ultrasonography versus contrast-enhanced ultrasonography for surveillance of hepatocellular carcinoma: a prospective multicenter randomized controlled trial. Liver Cancer 2019;8:271−280.ArticlePubMedPMCPDF
- 52. Park JH, Park MS, Lee SJ, Jeong WK, Lee JY, Park MJ, et al. Contrast-enhanced US with perfluorobutane for hepatocellular carcinoma surveillance: a multicenter diagnostic trial (SCAN). Radiology 2019;292:638−646.ArticlePubMed
- 53. Tanaka H, Iijima H, Nouso K, Aoki N, Iwai T, Takashima T, et al. Cost-effectiveness analysis on the surveillance for hepatocellular carcinoma in liver cirrhosis patients using contrast-enhanced ultrasonography. Hepatol Res 2012;42:376−384.ArticlePubMed
- 54. Minami Y, Kudo M, Hatanaka K, Kitai S, Inoue T, Hagiwara S, et al. Radiofrequency ablation guided by contrast harmonic sonography using perfluorocarbon microbubbles (Sonazoid) for hepatic malignancies: an initial experience. Liver Int 2010;30:759−764.ArticlePubMed
- 55. Dohmen T, Kataoka E, Yamada I, Miura K, Ohshima S, Shibuya T, et al. Efficacy of contrast-enhanced ultrasonography in radiofrequency ablation for hepatocellular carcinoma. Intern Med 2012;51:1−7.ArticlePubMed
- 56. Lee MW, Lim HK, Rhim H, Cha DI, Kang TW, Song KD, et al. Percutaneous radiofrequency ablation of small (1-2 cm) hepatocellular carcinomas inconspicuous on B-mode ultrasonographic imaging: usefulness of combined fusion imaging with MRI and contrast-enhanced ultrasonography. Can J Gastroenterol Hepatol 2018;2018:7926923. ArticlePubMedPMCPDF
- 57. Inoue T, Kudo M, Hatanaka K, Arizumi T, Takita M, Kitai S, et al. Usefulness of contrast-enhanced ultrasonography to evaluate the post-treatment responses of radiofrequency ablation for hepatocellular carcinoma: comparison with dynamic CT. Oncology 2013;84 Suppl 1:51−57.ArticlePubMedPDF
- 58. Sugimoto K, Moriyasu F, Saito K, Rognin N, Kamiyama N, Furuichi Y, et al. Hepatocellular carcinoma treated with sorafenib: early detection of treatment response and major adverse events by contrastenhanced US. Liver Int 2013;33:605−615.ArticlePubMedPDF
- 59. Takizawa K, Numata K, Morimoto M, Kondo M, Nozaki A, Moriya S, et al. Use of contrast-enhanced ultrasonography with a perflubutane-based contrast agent performed one day after transarterial chemoembolization for the early assessment of residual viable hepatocellular carcinoma. Eur J Radiol 2013;82:1471−1480.ArticlePubMed
- 60. Funaoka A, Numata K, Takeda A, Saigusa Y, Tsurugai Y, Nihonmatsu H, et al. Use of contrast-enhanced ultrasound with sonazoid for evaluating the radiotherapy efficacy for hepatocellular carcinoma. Diagnostics (Basel) 2021;11:486. ArticlePubMedPMC
References
Figure & Data
References
Citations

- Sonazoid contrast-enhanced ultrasonography for the diagnosis of hepatocellular carcinoma: strengths and shortcomings
Sung Won Lee, Min Kyu Kang, Xiang Zhang
Journal of Liver Cancer.2023; 23(2): 238. CrossRef
 PubReader
PubReader ePub Link
ePub Link Download Citation
Download Citation
- Download Citation
- Close
- Related articles
-
- Multidisciplinary approach for hepatocellular carcinoma patients: current evidence and future perspectives
- Changing etiology and epidemiology of hepatocellular carcinoma: Asia and worldwide
- Current perspectives on radiotherapy in hepatocellular carcinoma management: a comprehensive review
- The efficacy of treatment for hepatocellular carcinoma in elderly patients

 E-submission
E-submission THE KOREAN LIVER CANCER ASSOCIATION
THE KOREAN LIVER CANCER ASSOCIATION


 Follow JLC on Twitter
Follow JLC on Twitter