Articles
- Page Path
- HOME > J Liver Cancer > Volume 23(2); 2023 > Article
-
Review Article
Imaging prognostication and tumor biology in hepatocellular carcinoma -
Diana Kadi1
 , Marilyn F. Yamamoto2
, Marilyn F. Yamamoto2 , Emily C. Lerner3
, Emily C. Lerner3 , Hanyu Jiang4
, Hanyu Jiang4 , Kathryn J. Fowler5
, Kathryn J. Fowler5 , Mustafa R. Bashir6,7,8
, Mustafa R. Bashir6,7,8
-
Journal of Liver Cancer 2023;23(2):284-299.
DOI: https://doi.org/10.17998/jlc.2023.08.29
Published online: September 15, 2023
1Department of Radiology, Duke University Medical Center, Durham, NC, USA
2Department of Radiology, Duke University School of Medicine, Durham, NC, USA
3Department of Radiology, Duke University School of Medicine, Durham, NC, USA
4Department of Radiology, West China Hospital, Sichuan University, Chengdu, China
5Department of Radiology, University of California San Diego, San Diego, CA, USA
6Department of Radiology, Duke University, Durham, NC, USA
7Division of Hepatology, Department of Medicine, Duke University, Durham, NC, USA
8Center for Advanced Magnetic Resonance Development, Duke University, Durham, NC, USA
-
Corresponding author: Mustafa R. Bashir, Department of Radiology; Division of Hepatology, Department of Medicine; Center for Advanced Magnetic Resonance Development, Duke University Medical Center, 10 Duke Medicine Cir, Durham, NC 27710, USA
Tel. +1-312-391-3990, Fax. +1-919-684-7128 E-mail: Mustafa.bashir@duke.edu
© 2023 The Korean Liver Cancer Association.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 2,009 Views
- 110 Downloads
Abstract
- Hepatocellular carcinoma (HCC) is the most common primary liver malignancy, and represents a significant global health burden with rising incidence rates, despite a more thorough understanding of the etiology and biology of HCC, as well as advancements in diagnosis and treatment modalities. According to emerging evidence, imaging features related to tumor aggressiveness can offer relevant prognostic information, hence validation of imaging prognostic features may allow for better noninvasive outcomes prediction and inform the selection of tailored therapies, ultimately improving survival outcomes for patients with HCC.
- Primary liver cancer is the sixth most prevalent cancer worldwide and the fourth leading cause of cancer-related mortality, with a 5-year survival rate of 18%.1 Hepatocellular carcinoma (HCC), which accounts for about 90% of primary liver cancers,2 typically arises in the setting of chronic liver disease and cirrhosis. Clinical practice guidelines such as that published by the Barcelona Clinic Liver Cancer (BCLC) incorporate tumor stage, underlying liver disease severity, potential transplant candidacy, and patient functional status as important factors to define management strategies, which range from curative options like transplantation or resection for early-stage disease to noncurative options like systemic therapy for advanced stage disease.3,4
- Despite treatment being selected based on tumor stage, the prognosis of HCC is heterogenous and remains poor with a median survival of 6-10 months and an average 5-year survival rate of 18% in the United States.1,5,6 Poor outcomes are attributed to challenges with early detection that lead to later stage at presentation, the scarcity of available transplant organs, and considerable tumor molecular heterogeneity.7 Fortunately, growing evidence suggests that imaging features related to tumor aggressiveness can provide prognostic information and might aid in the selection of tailored therapies.8 Currently, pathomolecular features are measurable only through biopsy or resection/transplantation. Biopsy is prone to sampling error and histopathology from surgery is unavailable at the time of initial treatment selection. While evidence is emerging that prognostic imaging features may hold value, further validation and understanding of the clinical utility of prognostic imaging features is needed. This review will focus on potential imaging prognostic features and their association with histological findings. Before delving into the imaging features, it is important to review the pathological and molecular phenotypes of HCC that form the current basis of our understanding of prognosis.
INTRODUCTION
- A major driver of biological heterogeneity in HCC is molecular heterogeneity. HCC demonstrates substantial molecular heterogeneity, both intertumorally and intratumorally. Intratumoral heterogeneity can lead to the development of tumor subpopulations with distinct risks of recurrence, metastatic capacity, and sensitivity to different therapies.9 Several reports have established a molecular and immune categorization of HCC based on genomic, epigenomic, histopathological, and immunological assessments.10-12 The most extended HCC molecular classification distinguishes between the proliferative class and the non-proliferative class and depending on the degree of immune cell infiltration there’s two main classes; inflamed or hot tumors and non-inflamed or cold tumors.
- Although we now have a better grasp of the molecular drivers of disease pathophysiology, there remains much to be discovered and clinical application of this information is yet to be achieved. While the most prevalent mutations (e.g., mutations in the TERT promoter, CTNNB1, and TP53) are not clinically actionable, 25% of HCCs contain potentially targetable driver changes (e.g., VEGF/VEGFR, GPC-3), which can be identified by next-generation sequencing studies.13-15 The use of these markers to predict response remains elusive however, for example studies show that the presence of programmed cell death protein 1 (PD-1)/programmed cell death ligand 1 (PD-L1) is not predictive of response to PD-1/PD-L1 checkpoint inhibition.
- Over the last decade, global efforts in HCC genetic subtyping along with examinations of the associated clinical, etiological, and histological aspects have greatly enhanced the sub-categorization of HCC, resulting in the identification of specific molecular classes. Specifically, HCCs are divided into two broad classes, the proliferative and non-proliferative classes, each of which includes various subclasses previously identified across multiple studies.16-19 These two classes are associated with distinct liver disease etiologies, clinical and pathological features, and patient outcomes, and so may be useful in prognostication.11
- Along with molecular classes, HCCs can be categorized into pathological subtypes. Approximately 35% of HCC can be classified into one of eight distinct pathological subtypes (i.e., macrotrabecular massive, steatohepatitic, scirrhous, fibrolamellar, chromophobe, lymphocyte-rich, clear cell and neutrophil-rich), while the remainder referred to as not-otherwise specified HCC. In previous World Health Organization classifications, combined hepatocellular-cholangiocarcinoma was also considered a sub-type of HCC; however, this is now recognized as a distinct entity. Though the relationship between these classification systems is not well established, generally the clear cell, sarcomatoid, pleomorphic, scirrhous, and macrotrabecular tumors are considered to belong to the proliferative class, while steatohepatitic and microtrabecular tumors belong to the non-proliferative class.20 Assigning an HCC to a particular pathological subtype may be advantageous for selection of specific therapies, for example those that enhance the immune to tumors.
- 1. Proliferative class
- Proliferative tumors account for approximately 50% of HCCs and are diverse, with considerable abundance in proliferation-related signaling pathways such as PI3K-AKT-mTOR, RAS-MAPK, and MET cascades.17,18,21,22 Chromosomal instability appears to be a driving factor in these tumors, with TP53 inactivation and FGF19 and/or CCND1 amplifications being particularly prevalent.23,24 This class also includes the majority of gene expression patterns linked to tumor recurrence,25 decreased survival time,26 and poor clinical outcomes. Several studies11,27,28 have suggested that there are two major subclasses within the proliferative class: a WNT-TGF β group (also known as S1 tumors) characterized by activation of non-canonical WNT; and a progenitor cell group (also known as S2 tumors) characterized by overexpression of epithelial cell adhesion molecule (EpCAM), alpha-fetoprotein (AFP), and insulin like growth factor-2, as well as a distinct DNA hypermethylation signature.29 Clinically, tumors of the proliferative class are more prevalent in hepatitis B virus-linked HCC, associated with higher levels of serum alpha-fetoprotein, increased aggressiveness, and a poorer prognosis.22
- A newly identified morphological variation of proliferative HCC is the macrotrabecular-massive HCC (MTM-HCC) subtype, which accounts for 5-15% of HCC and has poor prognosis due to increased invasiveness and metastatic dissemination.30-33 MTM-HCC is associated with substantial necrosis, low apparent diffusion coefficient (ADC) values, large size,34-36 and the vessel encapsulated tumor clusters pattern (VETC). VETC is a process through which tumors may enclose cells that have drifted and are in the blood stream in order to shield them from immune response and apoptosis. VETC is a negative prognostic factor associated with high AFP, larger size, worse grade, high rate of microvascular invasion (MVI) and few inflammatory infiltrates with a detrimental influence on survival.37
- The typical imaging appearance of other subtypes, such as scirrhous and neutrophil-rich HCC, remains poorly characterized. These HCCs may be distinguished by a greater prevalence of targetoid dynamic enhancement pattern (of the LR-M classification according to the Liver Imaging Reporting and Data System [LI-RADS] criteria), pronounced hepatobiliary phase (HBP) hypointensity, low ADC values, and non-smooth tumor margins.38-40
- 2. Non-proliferative class
- The non-proliferative class of HCC accounts for 50% of HCC.11,21 These tumors maintain hepatocyte-like characteristics, including activation of the canonical WNT signaling pathway, mostly through mutations in CTNNB1 (encoding β-catenin),11,41 and a greater rate of TERT promoter mutations. Within this molecular subgroup, CTNNB1-mutated HCCs are a homogeneous subtype with cholestasis, microtrabecular, and pseudoglandular architectural features. Clinically, non-proliferative class tumors are associated with specific etiologies of underlying liver disease including alcohol use, hepatitis C virus and nonalcoholic steatohepatitis.22 In comparison to the proliferative class, the non-proliferative class generally consists of less aggressive, well-differentiated tumors associated with lower levels of serum alpha-fetoprotein and better clinical outcomes. For instance, steatohepatitic HCC (SH-HCC), the most prevalent subtype (5-20% of HCCs), is characterized by smaller size and often contains fat.SH-HCC has a generally less aggressive character and has been associated with distinct molecular characteristics, including frequent IL-6-JAK-STAT pathway activation without changes in CTNNB1, TERT and TP53 pathways, lower histologic grade, less frequent MVI, fewer metastasis, as well as background nonalcoholic fatty liver disease and the metabolic syndrome.20,36,42,43
SUBTYPES OF HEPATOCELLULAR CARCINOMA
- There is growing evidence suggesting that certain imaging features have prognostic value and are correlated with histological factors that impact survival outcomes (Table 1). Integration of imaging prognostic features in clinical management algorithms may help tailor treatment strategies for patient with HCC. For example, patients with early-stage HCC and imaging features portending a good prognosis may be candidates for less aggressive curative-intent treatment options, while those with poor prognostic features may benefit from more aggressive treatments (e.g., surgery over ablation or wide-margin over narrow-margin resection). Thus, prognostic imaging features have the potential to serve as non-invasive decision-making biomarkers for optimizing treatment selection for HCC patients. The following sections describe several novel prognostic imaging features and the current evidence.
- 1. Tumor margin
- Tumor margin refers to the outer contour of the mass or lesion of interest. Tumor margin may be described as either smooth or irregular. Tumors with smooth margins are well-circumscribed about the entirety of the tumor, lacking irregularities or projections. These tumors are typically histologically encapsulated with lower frequency of invasion into the surrounding liver parenchyma.44 Previous work has found that smooth tumor margins are associated with non-proliferative subtypes of HCC.45 In contrast, irregular tumor margins, which are at least partially nodular or infiltrative, have been associated with proliferative HCCs, particularly the progenitor as well as the macrotrabecular-massive (MTM) subtypes.46 The irregular margins are thought to be a consequence of the aggressive growth pattern characteristic of proliferative HCCs.39,45,47 Poorly differentiated HCC can display stem cell markers such cytokeratin 19 or EpCAM.48 Choi et al.40 observed that irregular margin on gadoxetic acid–enhanced HBP images is associated with cytokeratin 19 positivity, which portends early recurrence rates and high mortality. Further, irregular tumor margins on pre-operative computed tomography (CT) and magnetic resonance imaging (MRI) have been repeatedly shown to be strong indicators of MVI.44,46,49 Patients with MVI have higher recurrence rates and lower survival after resection compared to those without MVI.39,46,47,50,51 Tumors with irregular margins and parenchymal infiltration at imaging are more likely to demonstrate histologically infiltrative growth, tumor in the vein, and extrahepatic metastasis.52,53
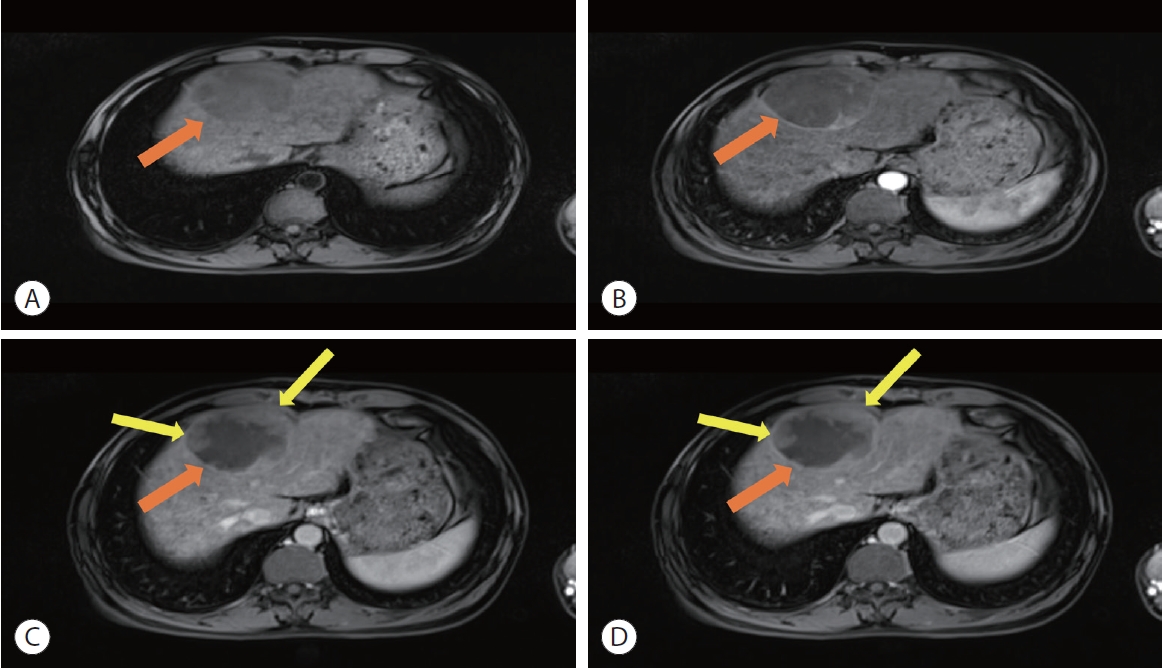
- 2. Hepatobiliary phase peritumoral hypointensity
- HBP peritumoral hypointensity, a “wedge-shaped” or “flame-like” region of low signal intensity outside the tumor margin on HBP MRI, has been associated with poor patient outcomes.54,55 Previous work has shown that HBP peritumoral hypointensity is highly correlated with MVI.55,56 Kim et al.55 suggested that HBP peritumoral hypointensity without associated signal intensity changes on non-HBP sequences represents the change in function of hepatocytes surrounding the tumor. This explains why this imaging finding has a high specificity yet low sensitivity for MVI, as altered hepatocyte function is thought to occur later in the course of MVI development and expansion.55 Chen et al.57 showed that hemodynamic alterations due to tumoral obstruction of portal venules lead to decreased organic anion transport polypeptide 1B3 (OATP1B3), a contributor to carcinogenesis and poor prognostic indicator. OATP1B3 is a transport ion involved in the uptake of bile salts and aids in the uptake of hepatocellular contrast agents.52 Accordingly, reduced expression of OATP1B3 is associated with decreased intensity in the HBP. Previous studies have shown that patients with tumors demonstrating HBP peritumoral hypointensity had higher recurrence rates after treatment and poorer overall survival than those without peritumoral HBP findings.58,59 Kang et al.60 demonstrated that rate of local tumor progression was higher in patients with HBP peritumoral hypointensity after radiofrequency ablation, likely due to the presence of MVI.60,61
- 3. Peritumoral arterial phase hyperenhancement
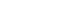
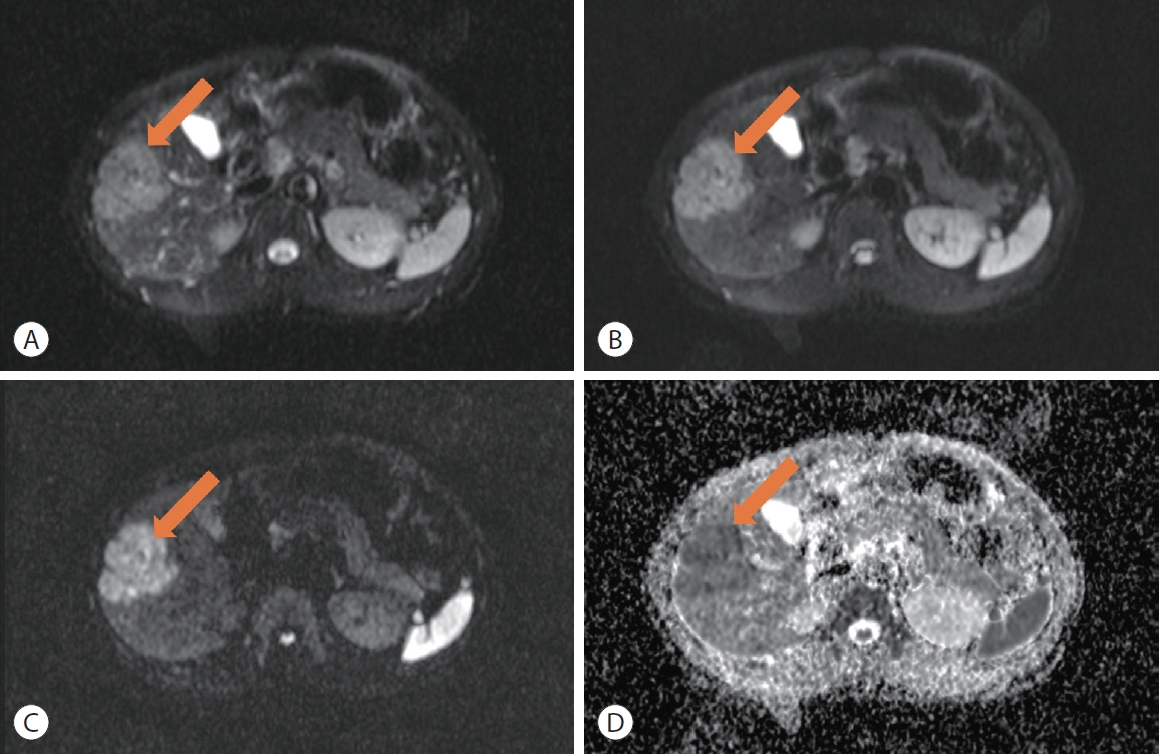
- Hyperenhancement of liver parenchyma adjacent to a tumor in the arterial phase with fade (isointensity or near isointensity) in the later post-contrast phases has been shown to be an independent risk factor for higher pathological grade.62 When occurring in the early arterial phase, this finding is associated with MVI (Fig. 1).52,54,62,63 Though the pathophysiology of this phenomenon is unclear, it is hypothesized to be due to tumor obstruction of venules leading to direct connections between tumor and hepatic sinusoids, or alternatively compensatory arterial supply from reduced venous flow.52,64 Regardless, patients with early peritumoral hyperenhancement have a worse prognosis with higher rates of early recurrence.65
- When peritumoral hyperenhancement occurs in the late arterial phase, it is described as “coronal enhancement” and has been linked to early draining of contrast from the tumor sinusoids or portal venules (Fig. 2).54 These drainage pathways carry contrast to the peritumoral liver parenchyma, resulting in enhancement seconds after initial enhancement of the tumor itself.66 Tumors demonstrating corona enhancement are typically hypervascular, and the finding is a poor prognostic indicator most closely associated with the macrotrabecular massive subtype of HCC.52,67 Large or irregularly shaped corona enhancement is also associated with MVI and recurrence in the form of satellite nodules after resection or ablation.66 Li et al.67 showed that in non-metastatic tumors with corona enhancement, liver resection provided greater survival benefit over transcatheter arterial chemoembolization (TACE), showing the clinical implications of this imaging finding.
- 4. Hepatobiliary phase intensity
- In primary hepatocellular tumors, hepatobiliary contrast agent uptake is related to the number and function of organic anion transport polypeptide (OATP) receptors and has been correlated with the degree of tumor differentiation.68 HCC may be hypointense or iso/hyperintense compared to the liver parenchyma in the HBP.
- Specifically, HCCs with HBP iso/hyperintensity tend to be well-differentiated and are associated with favorable survival outcomes.69 HBP hyperintensity has been associated with increased expression of beta-catenin, which increases expression of OATP1B3, as demonstrated by Kitao et al..70 HBP hyperintense HCCs have also been associated with increased production of hepatocyte nuclear factor 4-alpha, which suppresses hepatocyte proliferation and HCC expansion.71,72 Histologically, these tumors are well to moderately differentiated.71 Kim et al.73 also showed that hyperintense tumors on HBP have lower rates of MVI and less likely to show infiltrative or scirrhous patterns on histology. According to the LI-RADS 2018, lesions that appear isointense relative to the liver are likely to be benign.74 Approximately 8% of HCCs appear isointense on HBP imaging, primarily those of the non-proliferative subtypes.52,75 Park et al.76 demonstrated that like hyperintense lesions, isointense lesions are associated with lower histological grade and typically well-differentiated.
- HBP hypointensity has been shown to be a sensitive diagnostic feature of HCC.77 HBP hypointense HCCs have a worse prognosis and increased likelihood for post-surgical tumor recurrence compared with the HBP iso/hyperintense ones.78 As with peritumoral HBP hypointensity, tumor hypointensity has also been associated with MVI.52 The degree of HBP hypointensity can also provide insight into the aggressiveness of HCC. Mild hypointensity is present when lesion intensity in the HBP is lower than that of liver but higher than that of vessels and a sign of better tumor differentiation. Marked hypointensity, where lesion intensity in the HBP is lower than that of liver and similar to or lower than that of intrahepatic vessels, associated with poorly-differentiated tumors or the progenitor-type HCC.39,52 Moreover, patients with HBP hypointense lesions typically have higher levels of AFP and a higher likelihood of portal venous invasion than those with HBP hyperintense lesions.79
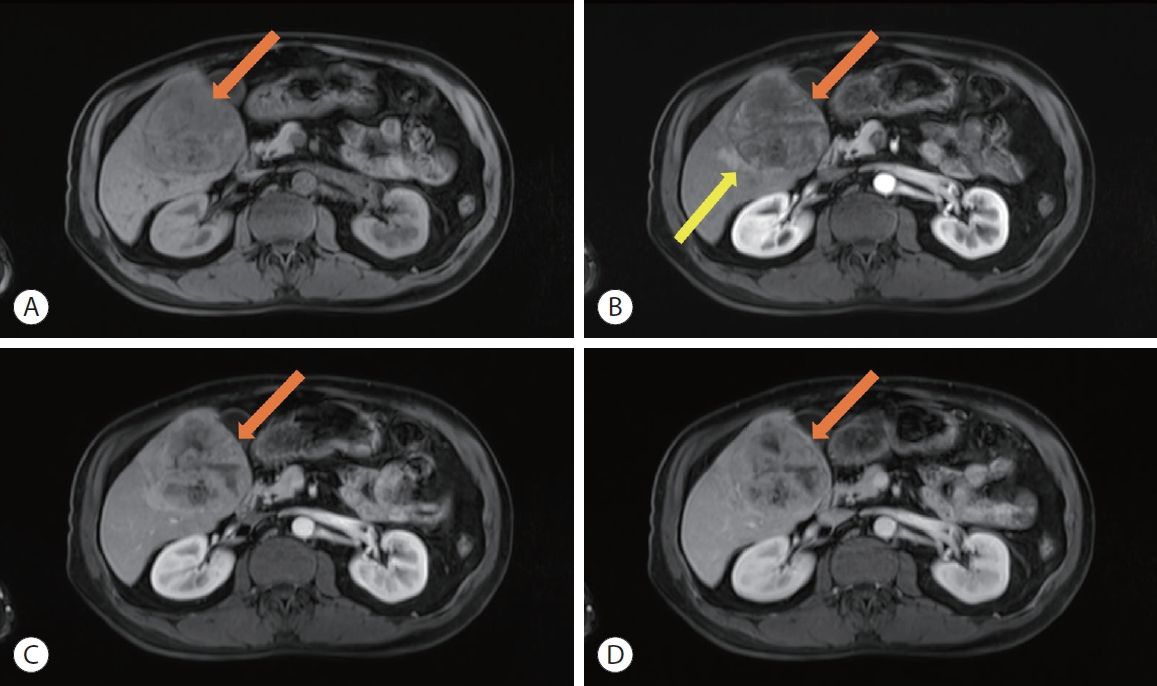
- 5. Tumor in bile duct
- The presence of tumor within bile duct can be secondary to HCC, intrahepatic cholangiocarcinoma, or combined tumors (Fig. 3). In HCC, bile duct invasion is less common than vascular invasion and is not well characterized in the literature.52 Even the specific constellation of imaging findings that define the presence of tumor in bile duct (including enhancing soft tissue within a bile duct and ductal dilation) have not been precisely defined. Jang et al.80 demonstrated that bile duct invasion most frequently occurred in the presence of MVI and is associated with higher mortality. However, bile duct invasion remains controversial as a prognostic indicator, with some studies suggesting that prognosis is similar between those with and without bile duct involvement.81 Jang et al.80 described that the finding of bile duct invasion may be best used as a prognostic indicator in early-stage HCC. Navadgi et al.82 conducted a systematic review which showed that patients with bile duct involvement had more advanced stage HCC with poor histologic differentiation and inferior survival following resection. It has been previously demonstrated that capsule infiltration, intrahepatic metastasis, and portal venous invasion were associated with bile duct invasion, showing the infiltrative tendency of these tumors.83
- Multiple hypotheses have been proposed to explain the relatively poor prognosis associated with bile duct invasion, though with little known about the molecular pathways driving this process, there is little consensus in the literature.80,82 Qin et al.84 demonstrated the utility of detecting this finding, as early detection and intervention can improve patient outcomes. Additionally, appropriate treatment selection may benefit from differentiating HCC from cholangiocarcinoma as the source of tumor in bile duct. Despite sharing some imaging features, HCC bile duct invasion tends to exhibit arterial phase hyperenhancement with early washout in the portal venous phase on CT, while cholangiocarcinoma shows irregular wall thickening and narrowing of the bile duct lumen.83,85
- 6. Tumor ischemia and necrosis
- The imaging finding ischemia describes as an area within a solid mass that enhances slowly or not at all, when compared to the rest of the mass (Fig. 4). This should not be confused with intralesional hemorrhage or hypoenhancement following treatment of a lesion. When present in HCC, ischemia is associated with MVI.36 However, this finding can be difficult to assess in lesions with nodule-in-nodule or mosaic appearance, as various compartments/nodules may enhance differently in the absence of ischemia.86 Ischemia can also be difficult to assess in lesions containing substantial amounts of fat or iron due to attendant signal intensity or attenuation alternations, particularly if imaging was performed in a single or limited dynamic phase. Further, differentiation of ischemia from fibrosis within a mass may not be possible on imaging alone and may require histologic assessment.
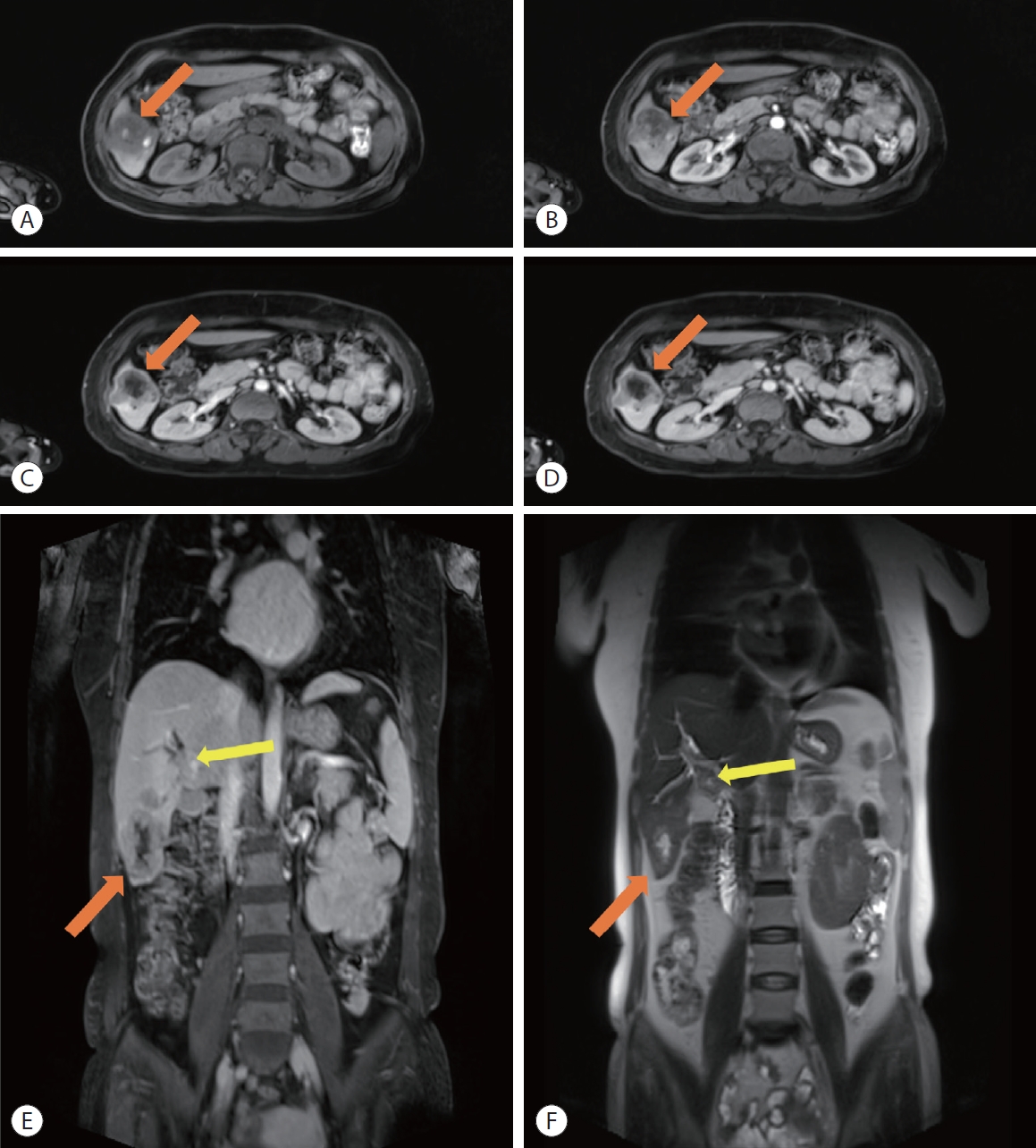
- Necrosis is characterized by the complete lack of contrast enhancement within a solid mass and marked T2 hyperintensity not attributable to cystic change (Fig. 5). Previous studies have shown that necrosis in the absence of prior treatment or hemorrhage is associated with larger, proliferative forms of HCC, specifically the MTM subtype.8,34,87 Mulé et al.34 showed that the presence of substantial necrosis, defined as necrosis occuping ≥20% of the tumor, was associated with both early (within 2 years) and overall likelihood of HCC recurrance after resection. These regions of necrosis can be found in rapidly expanding tumors due to hypoxia, as described by Tohme et al..88 Extensive cellular hypoxia has been associated with increased metastatic potential and poorer sensitivity to radiotherapy and chemotherapy.89 Wang et al.90 demonstrated that tumor micronecrosis (tumor necrosis that can only be observed histologically) can independently predict decreased recurrance free and overall survival after liver transplantation, indicating the need for close follow up after treatment. Moreover, the histologic subtype of necrosis (e.g., neutrophil-rich, scirrhous and lymphocyte-rich) has been shown to influence prognosis after TACE, with liquefactive necrosis associated with increased rate of recurrence and metastasis.91
- 7. Diffusion restriction and low ADC value
- Diffusion restriction is characterized by increased intensity of the tumor in comparison to the surrounding liver parenchyma on high b-value (≥800 s/mm2) diffusion-weighted imaging that is not caused by T2 the shine-through effect (Fig. 6). Diffusion restriction may be mild-moderate, where the mass is of higher signal intensity than the surrounding liver but less than that of the non-iron-overloaded spleen, or marked, where the mass is of higher signal intensity than both the liver and non-iron-overloaded spleen.8,52,66 Diffusion restriction is typically related to increased cellularity, increased nucleus-to-cytoplasm ratio, and decreased extracellular matrix, all of which lead to decreased random water diffusion at the molecular scale.52 When present in a focal lesion, diffusion restriction can be suggestive of malignancy. However, it lacks specificity for HCC as some HCCs do not restrict diffusion, particularly those that are well-differentiated, and other tumor types such as cholangiocarcinoma may restrict diffusion.40,66 Despite this, Jiang et al.86 previously demonstrated that marked diffusion restriction on MRI was associated with MVI. However, caution should be exercised as a recent meta-analysis showed that differences in the strength of association between diffusion restriction and MVI can be attributed at least in part to variability in field strength, MRI system manufacturer, and model.92 Generally, a clear consensus has not emerged in the literature regarding prognostic differences between mild-moderately vs. markedly restricted diffusion.
- Quantitative analysis of diffusion restriction can be performed using ADC values. On this basis, a mass with an ADC value lower than that of the liver parenchyma is said to restrict diffusion. A mildly-moderately low ADC value is lower than that of the liver but higher than that of the spleen, while a markedly low ADC value is lower than that of both organs.8 Previous studies have shown that poorly differentiated tumors have lower ADC value than well-differentiated lesions.52,92-94 Chang et al.94 demonstrated that ADC value alone was able to accurately identify HCC histologic grade with a specificity of 89.8%. Furthermore, Mori et al.93 showed that tumors with lower ADC values were more likely to demonstrate portal vein invasion with increased metastatic potential. Lee et al.95 confirmed this theme by showing that low ADC was correlated with increased MVI and early recurrence after surgical resection. It remains unclear whether ADC value has greater prognostic value than categorical assessment of the presence or absence or restricted diffusion.
- 8. Multifocality
- Multifocal HCC occurs in approximately one-third of patients and can reflect multicentric disease (multiple tumors arising independently of one another) or metachronous intrahepatic metastases from a single dominant tumor.52,96 Tumors of multicentric origin tend to respond well to locoregional therapy while those that arise metachronously tend to recur earlier after curative resection.97 Patients with intrahepatic metastases also have a poorer prognosis.96 This is suspected to be due to each tumor having increased metastatic potential and likelihood of vascular invasion.52,92 Choi et al.40 found that multifocal HCC is associated with cytokeratin 19 positivity, worse histologic grade, and early recurrence. Yue and Zhou98 determined that the extent of tumor involvement of the liver or nearby organs, elevated AFP, and large size were each independent predictors for worse prognosis in patients with multifocal HCC.
- Currently, imaging methods for differentiating the two types of multifocal HCC have not been validated.66 However, some imaging characteristics can be useful for distinguishing between subtypes. Multicentric HCCs tend to have a nodule-in-nodule appearance or nodules with different imaging characteristics.52 In contrast, intrahepatic metastatic disease tends to have satellite nodule pattern and similar imaging characteristics between the dominant lesion and smaller lesions.52,66 Satellite nodules indicate tumor capsular invasion and are typically seen in progressed HCCs.66 Overall, multifocal HCC presents a challenge regarding treatment, as surgery requires wide resection margins or multiple surgeries to achieve complete tumor removal, increasing the risk for liver failure.99 Despite this, Risaliti et al.99 found that hepatic resection is a viable option in patients with multifocal disease, providing longer disease-free survival than TACE though similar overall survival. Supporting this, Yue and Zhou98 found that hepatic resection provided improved long-term survival when compared with radiofrequency ablation. Multifocal HCC has a negative prognostic value and is associated with more aggressive disease and a higher risk of tumor recurrence and metastases.
PROGNOSTIC IMAGING FEATURES
- Although there is a burgeoning body of literature supporting the use of prognostic imaging features, a number of limitations must be acknowledged. First, consensus definitions for the types and levels of these features are only now emerging, creating challenges in identifying patterns between prior works. In addition, most of the existing literature is retrospective and single-center in nature, and the repeatability and reproducibility in determining the presence of these features has not been thoroughly assessed. Many publications lack rigorous radiologic-pathologic correlation, and correlate these features with short-term pathological outcomes such as MVI and tumor grade, rather than more clinically relevant survival outcomes. Nonetheless, even with experienced radiologists, there is a notable inter-reader inconsistency in the evaluation of imaging prognostic features such as MVI.100
- Despite these limitations, the fact that imaging features have been associated with a variety of outcomes at different centers provides encouraging evidence that these features may be generalizable. The poor prognosis for HCC and emergence of new therapies underscores the need for new tools to enable patient-specific treatment selection. Given this need, more rigorous multi-center investigations are needed into the potential role for prognostic imaging features to fill this role.
CHALLENGES AND FUTURE DIRECTIONS
- Prognosis of HCC, mainly limited to tumor stage, is critical for treatment selection. Noninvasive imaging techniques are essential for surveillance, diagnosis, characterization and staging of HCC tumors. Nevertheless, prognostic pathomolecular features correlated to biological aggressiveness are currently only assessed through invasive exams. Emerging efforts are pointing towards validation of imaging prognostic features as non-invasive driver of outcomes. These findings may lead to translating our current understanding of the biology of HCC into clinical use, enhancing precision medicine for patients with this highly aggressive tumor.
CONCLUSION
-
Conflict of Interest
The authors have no conflicts of interest to disclose.
-
Ethics Statement
This review article is fully based on articles which have already been published and did not involve additional patient participants. Therefore, IBR approval is not necessary.
-
Funding Statement
None.
-
Data Availability
No data were generated or analyzed during the study.
-
Author Contribution
Writing–original draft: DK, MFY
Writing–review & editing: ECL, HJ, KJF, MRB
Article information


| Imaging feature | Definition | Pathomolecular associations and outcomes | References | |
|---|---|---|---|---|
| Better prognosis | ||||
| Smooth tumor margin | Tumor margin, in its entirety, is uninterrupted, free from irregularities or projections, and well-defined | Nonproliferative subtypes | 44, 45, 101 | |
| Encapsulated tumors, lower frequency of invasion into the surrounding liver parenchyma | ||||
| HBP iso/hyper intensity | HBP isointensity, when lesion intensity in the HBP is nearly identical to liver | Well to moderately-differentiated tumors, lower rates of MVI | 8, 69-76 | |
| HBP hyperintensity, when lesion intensity in the HBP is higher than that of the liver | Less likely to show infiltrative or scirrhous patterns on histology | |||
| Increased expression of beta-catenin, which increases expression of OATP1B3; increased production of HNF4- alpha, which suppresses hepatocyte proliferation and HCC expansion | ||||
| Worse prognosis | ||||
| Non-smooth tumor margins | Tumor margin, at least in part, is irregular and/or has areas of bulging, nodular projection, or infiltration into adjacent tissues | MVI, proliferative subtypes, particularly progenitor and MTM subtypes | 39, 40, 46-53, 102 | |
| Infiltrative growth pattern, tumor in vein, and extrahepatic metastasis | ||||
| CK19 positivity | ||||
| HBP hypointensity | Mild HBP hypointensity, when lesion intensity in the HBP is lower than that of liver but higher than that of vessels | MVI, higher AFP levels, higher likelihood of tumor in vein | 8, 39, 52, 78, 79 | |
| Marked HBP hypointensity when lesion intensity in the HBP is lower than that of liver and similar to or lower than the vessels in the liver | Degree of HBP hypointensity is related to tumor differentiation; mild HBP hypointensity associated with better tumor differentiation marked HBP hypointensity associated with poorly-differentiated tumors and progenitor type HCC | |||
| Tumor in bile duct | Presence of tumor in bile duct lumen | MVI, advanced stage at presentation, poor histologic differentiation | 80, 82, 83 | |
| Capsular invasion, intrahepatic metastasis, and tumor in vein | ||||
| HBP peritumoral hypointensity | Non mass like hypointensity of liver adjacent to a mass in the hepatobiliary phase | MVI | 54-56, 60, 61 | |
| Higher recurrence rates after treatment, poorer overall survival | ||||
| Peritumoral hyperenhancement | Non-mass like area of liver adjacent to a mass with hyperenhancement in the arterial phase and fade in the postarterial phases | MVI, higher pathologic grade, MTM subtype | 52, 54, 62, 63, 65, 67 | |
| High rates of early recurrence post-treatment | ||||
| Tumor ischemia and necrosis | Slowly enhancing (ischemia) or nonenhancing (necrosis) area in a solid mass, not attributable to cystic component, prior treatment or intralesional hemorrhage | MVI, proliferative subtypes especially MTM | 8, 34, 87, 89-91 | |
| Increased metastatic potential, poorer sensitivity to radiotherapy and chemotherapy | ||||
| Diffusion restriction and low ADC value | Signal intensity higher than that of liver on high b-value diffusion-weighted images (e.g., b≥400 s/mm2), not caused only by T2 shine-through | Increased cellularity, increased nucleus-to-cytoplasm ratio, and decreased extracellular matrix | 8, 40, 52, 66, 86, 92-95 | |
| ADC value lower than or similar to liver, synonymous with diffusion restriction | MVI, poor differentiation, tumor in vein, increased metastatic potential | |||
| Low ADC associated with early recurrence after resection | ||||
| Multifocal HCC | Multiple observations in the liver that, in aggregate, are interpreted as advanced HCC | Increased extrahepatic metastatic potential, MVI CK19 positivity, worse grade, early recurrence | 40, 52, 66, 92, 96-99, 103 | |
- 1. Jemal A, Ward EM, Johnson CJ, Cronin KA, Ma J, Ryerson B, et al. Annual report to the nation on the status of cancer, 1975-2014, featuring survival. J Natl Cancer Inst 2017;109:djx030. ArticlePubMedPMC
- 2. Wittekind C. Pathology of liver tumors. Zentralbl Chir 2000;125:587−591.PubMed
- 3. Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, GarciaCriado Á, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol 2022;76:681−693.ArticlePubMedPMC
- 4. Llovet JM, De Baere T, Kulik L, Haber PK, Greten TF, Meyer T, et al. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2021;18:293−313.ArticlePubMedPDF
- 5. Brar G, Greten TF, Graubard BI, McNeel TS, Petrick JL, McGlynn KA, et al. Hepatocellular carcinoma survival by etiology: a SEERmedicare database analysis. Hepatol Commun 2020;4:1541−1551.ArticlePubMedPMCPDF
- 6. Goutté N, Sogni P, Bendersky N, Barbare JC, Falissard B, Farges O. Geographical variations in incidence, management and survival of hepatocellular carcinoma in a Western country. J Hepatol 2017;66:537−544.ArticlePubMed
- 7. Trevisani F, Garuti F, Neri A. Alpha-fetoprotein for diagnosis, prognosis, and transplant selection. Semin Liver Dis 2019;39:163−177.ArticlePubMed
- 8. Ronot M, Chernyak V, Burgoyne A, Chang J, Jiang H, Bashir M, et al. Imaging to predict prognosis in hepatocellular carcinoma: current and future perspectives. Radiology 2023;307:e221429.ArticlePubMed
- 9. Gerlinger M, Rowan AJ, Horswell S, Math M, Larkin J, Endesfelder D, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 2012;366:883−892.ArticlePubMedPMC
- 10. Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2016;2:16018. ArticlePubMedPDF
- 11. Zucman-Rossi J, Villanueva A, Nault JC, Llovet JM. genetic landscape and biomarkers of hepatocellular carcinoma. Gastroenterology 2015;149:1226−1239. e4.ArticlePubMed
- 12. Rebouissou S, Nault JC. Advances in molecular classification and precision oncology in hepatocellular carcinoma. J Hepatol 2020;72:215−229.ArticlePubMed
- 13. Llovet JM, Montal R, Sia D, Finn RS. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol 2018;15:599−616.ArticlePubMedPDF
- 14. Sangro B, Sarobe P, Hervás-Stubbs S, Melero I. Advances in immunotherapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2021;18:525−543.ArticlePubMedPMCPDF
- 15. Couri T, Pillai A. Goals and targets for personalized therapy for HCC. Hepatol Int 2019;13:125−137.ArticlePubMedPDF
- 16. Cancer Genome Atlas Research Network. Comprehensive and integrative genomic characterization of hepatocellular carcinoma. Cell 2017;169:1327−1341. e23.PubMedPMC
- 17. Hoshida Y, Nijman SM, Kobayashi M, Chan JA, Brunet JP, Chiang DY, et al. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer Res 2009;69:7385−7392.ArticlePubMedPMCPDF
- 18. Boyault S, Rickman DS, de Reyniès A, Balabaud C, Rebouissou S, Jeannot E, et al. Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology 2007;45:42−52.ArticlePubMed
- 19. Lee JS, Chu IS, Heo J, Calvisi DF, Sun Z, Roskams T, et al. Classification and prediction of survival in hepatocellular carcinoma by gene expression profiling. Hepatology 2004;40:667−676.ArticlePubMed
- 20. Calderaro J, Couchy G, Imbeaud S, Amaddeo G, Letouzé E, Blanc JF, et al. Histological subtypes of hepatocellular carcinoma are related to gene mutations and molecular tumour classification. J Hepatol 2017;67:727−738.ArticlePubMed
- 21. Chiang DY, Villanueva A, Hoshida Y, Peix J, Newell P, Minguez B, et al. Focal gains of VEGFA and molecular classification of hepatocellular carcinoma. Cancer Res 2008;68:6779−6788.ArticlePubMedPMCPDF
- 22. Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2021;7:6. ArticlePubMedPDF
- 23. Wang K, Lim HY, Shi S, Lee J, Deng S, Xie T, et al. Genomic landscape of copy number aberrations enables the identification of oncogenic drivers in hepatocellular carcinoma. Hepatology 2013;58:706−717.ArticlePubMed
- 24. Seehawer M, Heinzmann F, D’Artista L, Harbig J, Roux PF, Hoenicke L, et al. Necroptosis microenvironment directs lineage commitment in liver cancer. Nature 2018;562:69−75.ArticlePubMedPMCPDF
- 25. Villanueva A, Hoshida Y, Battiston C, Tovar V, Sia D, Alsinet C, et al. Combining clinical, pathology, and gene expression data to predict recurrence of hepatocellular carcinoma. Gastroenterology 2011;140:1501−1512.e2.ArticlePubMedPMC
- 26. Nault JC, De Reyniès A, Villanueva A, Calderaro J, Rebouissou S, Couchy G, et al. A hepatocellular carcinoma 5-gene score associated with survival of patients after liver resection. Gastroenterology 2013;145:176−187.ArticlePubMed
- 27. Llovet JM, Villanueva A, Lachenmayer A, Finn RS. Advances in targeted therapies for hepatocellular carcinoma in the genomic era. Nat Rev Clin Oncol 2015;12:408−424.ArticlePubMedPDF
- 28. Hoshida Y, Toffanin S, Lachenmayer A, Villanueva A, Minguez B, Llovet JM. Molecular classification and novel targets in hepatocellular carcinoma: recent advancements. Semin Liver Dis 2010;30:35−51.ArticlePubMedPMC
- 29. Villanueva A, Portela A, Sayols S, Battiston C, Hoshida Y, MéndezGonzález J, et al. DNA methylation-based prognosis and epidrivers in hepatocellular carcinoma. Hepatology 2015;61:1945−1956.ArticlePubMedPDF
- 30. Ziol M, Poté N, Amaddeo G, Laurent A, Nault JC, Oberti F, et al. Macrotrabecular-massive hepatocellular carcinoma: a distinctive histological subtype with clinical relevance. Hepatology 2018;68:103−112.ArticlePubMedPDF
- 31. Jeon Y, Benedict M, Taddei T, Jain D, Zhang X. Macrotrabecular hepatocellular carcinoma: an aggressive subtype of hepatocellular carcinoma. Am J Surg Pathol 2019;43:943−948.PubMed
- 32. Calderaro J, Meunier L, Nguyen CT, Boubaya M, Caruso S, Luciani A, et al. ESM1 as a marker of macrotrabecular-massive hepatocellular carcinoma. Clin Cancer Res 2019;25:5859−5865.ArticlePubMedPDF
- 33. Kumar D, Hafez O, Jain D, Zhang X. Can primary hepatocellular carcinoma histomorphology predict extrahepatic metastasis? Hum Pathol 2021;113:39−46.ArticlePubMed
- 34. Mulé S, Galletto Pregliasco A, Tenenhaus A, Kharrat R, Amaddeo G, Baranes L, et al. Multiphase liver MRI for identifying the macrotrabecular-massive subtype of hepatocellular carcinoma. Radiology 2020;295:562−571.ArticlePubMed
- 35. Chen J, Xia C, Duan T, Cao L, Jiang H, Liu X, et al. Macrotrabecular-massive hepatocellular carcinoma: imaging identification and prediction based on gadoxetic acid-enhanced magnetic resonance imaging. Eur Radiol 2021;31:7696−7704.ArticlePubMedPDF
- 36. Cannella R, Dioguardi Burgio M, Beaufrère A, Trapani L, Paradis V, Hobeika C, et al. Imaging features of histological subtypes of hepatocellular carcinoma: implication for LI-RADS. JHEP Rep 2021;3:100380. ArticlePubMedPMC
- 37. Renne SL, Woo HY, Allegra S, Rudini N, Yano H, Donadon M, et al. Vessels encapsulating tumor clusters (VETC) is a powerful predictor of aggressive hepatocellular carcinoma. Hepatology 2020;71:183−195.ArticlePubMedPDF
- 38. Loy LM, Low HM, Choi JY, Rhee H, Wong CF, Tan CH. Variant hepatocellular carcinoma subtypes according to the 2019 WHO classification: an imaging-focused review. AJR Am J Roentgenol 2022;219:212−223.ArticlePubMed
- 39. Chen J, Wu Z, Xia C, Jiang H, Liu X, Duan T, et al. Noninvasive prediction of HCC with progenitor phenotype based on gadoxetic acid-enhanced MRI. Eur Radiol 2020;30:1232−1242.ArticlePubMedPDF
- 40. Choi SY, Kim SH, Park CK, Min JH, Lee JE, Choi YH, et al. Imaging features of gadoxetic acid-enhanced and diffusion-weighted MR imaging for identifying cytokeratin 19-positive hepatocellular carcinoma: a retrospective observational study. Radiology 2018;286:897−908.ArticlePubMed
- 41. Lachenmayer A, Alsinet C, Savic R, Cabellos L, Toffanin S, Hoshida Y, et al. Wnt-pathway activation in two molecular classes of hepatocellular carcinoma and experimental modulation by sorafenib. Clin Cancer Res 2012;18:4997−5007.ArticlePubMedPMCPDF
- 42. Salomao M, Yu WM, Brown RS Jr, Emond JC, Lefkowitch JH. Steatohepatitic hepatocellular carcinoma (SH-HCC): a distinctive histological variant of HCC in hepatitis C virus-related cirrhosis with associated NAFLD/NASH. Am J Surg Pathol 2010;34:1630−1636.PubMed
- 43. Calderaro J, Ziol M, Paradis V, Zucman-Rossi J. Molecular and histological correlations in liver cancer. J Hepatol 2019;71:616−630.ArticlePubMed
- 44. Hwang YJ, Bae JS, Lee Y, Hur BY, Lee DH, Kim H. Classification of microvascular invasion of hepatocellular carcinoma: correlation with prognosis and magnetic resonance imaging. Clin Mol Hepatol 2023;29:733−746.ArticlePubMedPMCPDF
- 45. Liu G, Ma D, Wang H, Zhou J, Shen Z, Yang Y, et al. Three-dimensional multifrequency magnetic resonance elastography improves preoperative assessment of proliferative hepatocellular carcinoma. Insights Imaging 2023;14:89. ArticlePubMedPMCPDF
- 46. Hu H, Zheng Q, Huang Y, Huang XW, Lai ZC, Liu J, et al. A nonsmooth tumor margin on preoperative imaging assesses microvascular invasion of hepatocellular carcinoma: a systematic review and meta-analysis. Sci Rep 2017;7:15375. ArticlePubMedPMCPDF
- 47. Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693−699.ArticlePubMed
- 48. Zhuo JY, Lu D, Tan WY, Zheng SS, Shen YQ, Xu X. CK19-positive hepatocellular carcinoma is a characteristic subtype. J Cancer 2020;11:5069−5077.ArticlePubMedPMC
- 49. Zhang L, Lin JB, Jia M, Zhang CC, Xu R, Guo L, et al. Clinical and imaging features preoperative evaluation of histological grade and microvascular infiltration of hepatocellular carcinoma. BMC Gastroenterol 2022;22:369. ArticlePubMedPMCPDF
- 50. Sumie S, Nakashima O, Okuda K, Kuromatsu R, Kawaguchi A, Nakano M, et al. The significance of classifying microvascular invasion in patients with hepatocellular carcinoma. Ann Surg Oncol 2014;21:1002−1009.ArticlePubMedPDF
- 51. Chou CT, Chen RC, Lin WC, Ko CJ, Chen CB, Chen YL. Prediction of microvascular invasion of hepatocellular carcinoma: preoperative CT and histopathologic correlation. AJR Am J Roentgenol 2014;203:W253−W259.ArticlePubMed
- 52. Fowler KJ, Burgoyne A, Fraum TJ, Hosseini M, Ichikawa S, Kim S, et al. Pathologic, molecular, and prognostic radiologic features of hepatocellular carcinoma. Radiographics 2021;41:1611−1631.ArticlePubMed
- 53. Reynolds AR, Furlan A, Fetzer DT, Sasatomi E, Borhani AA, Heller MT, et al. Infiltrative hepatocellular carcinoma: what radiologists need to know. Radiographics 2015;35:371−386.ArticlePubMed
- 54. An C, Kim MJ. Imaging features related with prognosis of hepatocellular carcinoma. Abdom Radiol (NY) 2019;44:509−516.ArticlePubMedPDF
- 55. Kim KA, Kim MJ, Jeon HM, Kim KS, Choi JS, Ahn SH, et al. Prediction of microvascular invasion of hepatocellular carcinoma: usefulness of peritumoral hypointensity seen on gadoxetate disodiumenhanced hepatobiliary phase images. J Magn Reson Imaging 2012;35:629−634.ArticlePubMed
- 56. Wu Y, Zhu M, Liu Y, Cao X, Zhang G, Yin L. Peritumoral imaging manifestations on Gd-EOB-DTPA-enhanced MRI for preoperative prediction of microvascular invasion in hepatocellular carcinoma: a systematic review and meta-analysis. Front Oncol 2022;12:907076. ArticlePubMedPMC
- 57. Chen S, Li K, Jiang J, Wang X, Chai Y, Zhang C, et al. Low expression of organic anion-transporting polypeptide 1B3 predicts a poor prognosis in hepatocellular carcinoma. World J Surg Oncol 2020;18:127. ArticlePubMedPMCPDF
- 58. Bae JS, Kim JH, Lee DH, Kim JH, Han JK. Hepatobiliary phase of gadoxetic acid-enhanced MRI in patients with HCC: prognostic features before resection, ablation, or TACE. Eur Radiol 2021;31:3627−3637.ArticlePubMedPDF
- 59. Zhang Y, Wei H, Song B. Magnetic resonance imaging for treatment response evaluation and prognostication of hepatocellular carcinoma after thermal ablation. Insights Imaging 2023;14:87. ArticlePubMedPMCPDF
- 60. Kang TW, Rhim H, Lee J, Song KD, Lee MW, Kim YS, et al. Magnetic resonance imaging with gadoxetic acid for local tumour progression after radiofrequency ablation in patients with hepatocellular carcinoma. Eur Radiol 2016;26:3437−3446.ArticlePubMedPDF
- 61. Cucchetti A, Cescon M, Trevisani F, Pinna AD. Current concepts in hepatic resection for hepatocellular carcinoma in cirrhotic patients. World J Gastroenterol 2012;18:6398−6408.ArticlePubMedPMC
- 62. Rong D, Liu W, Kuang S, Xie S, Chen Z, Chen F, et al. Preoperative prediction of pathologic grade of HCC on gadobenate dimeglumine-enhanced dynamic MRI. Eur Radiol 2021;31:7584−7593.ArticlePubMedPDF
- 63. Hwang SH, Rhee H. Radiologic features of hepatocellular carcinoma related to prognosis. J Liver Cancer 2023;23:143−156.ArticlePubMedPMCPDF
- 64. Zhang J, Dong W, Li Y, Fu J, Jia N. Prediction of microvascular invasion in combined hepatocellular-cholangiocarcinoma based on preoperative contrast-enhanced CT and clinical data. Eur J Radiol 2023;163:110839. ArticlePubMed
- 65. Shan QY, Hu HT, Feng ST, Peng ZP, Chen SL, Zhou Q, et al. CTbased peritumoral radiomics signatures to predict early recurrence in hepatocellular carcinoma after curative tumor resection or ablation. Cancer Imaging 2019;19:11. ArticlePubMedPMCPDF
- 66. Choi JY, Lee JM, Sirlin CB. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part II. Extracellular agents, hepatobiliary agents, and ancillary imaging features. Radiology 2014;273:30−50.ArticlePubMedPMC
- 67. Li M, Xin Y, Fu S, Liu Z, Li Y, Hu B, et al. Corona enhancement and mosaic architecture for prognosis and selection between of liver resection versus transcatheter arterial chemoembolization in single hepatocellular carcinomas >5cm without extrahepatic metastases: an imaging-based retrospective study. Medicine (Baltimore) 2016;95:e2458.PubMedPMC
- 68. Kitao A, Matsui O, Yoneda N, Kozaka K, Shinmura R, Koda W, et al. The uptake transporter OATP8 expression decreases during multistep hepatocarcinogenesis: correlation with gadoxetic acid enhanced MR imaging. Eur Radiol 2011;21:2056−2066.ArticlePubMedPDF
- 69. Cho ES, Choi JY. MRI features of hepatocellular carcinoma related to biologic behavior. Korean J Radiol 2015;16:449−464.ArticlePubMedPMC
- 70. Kitao A, Matsui O, Yoneda N, Kozaka K, Kobayashi S, Sanada J, et al. Hepatocellular carcinoma with β-catenin mutation: imaging and pathologic characteristics. Radiology 2015;275:708−717.ArticlePubMed
- 71. Fujita N, Nishie A, Asayama Y, Ishigami K, Ushijima Y, Kakihara D, et al. Hyperintense liver masses at hepatobiliary phase gadoxetic acid-enhanced MRI: imaging appearances and clinical importance. Radiographics 2020;40:72−94.ArticlePubMed
- 72. Ning BF, Ding J, Yin C, Zhong W, Wu K, Zeng X, et al. Hepatocyte nuclear factor 4 alpha suppresses the development of hepatocellular carcinoma. Cancer Res 2010;70:7640−7651.PubMed
- 73. Kim JY, Kim MJ, Kim KA, Jeong HT, Park YN. Hyperintense HCC on hepatobiliary phase images of gadoxetic acid-enhanced MRI: correlation with clinical and pathological features. Eur J Radiol 2012;81:3877−3882.ArticlePubMed
- 74. American College of Radiology. Liver imaging reporting & data system (LI-RADS®) [Internet]. Reston (US): American College of Radiology [cited 2023 Jul 4]. Available from: https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS
- 75. Hui CL, Mautone M. Patterns of enhancement in the hepatobiliary phase of gadoxetic acid-enhanced MRI. Br J Radiol 2020;93:20190989. ArticlePubMedPMC
- 76. Park JH, Chung YE, Seo N, Choi JY, Park MS, Kim MJ. Hepatobiliary phase signal intensity: a potential method of diagnosing HCC with atypical imaging features among LR-M observations. PLoS One 2021;16:e0257308.ArticlePubMedPMC
- 77. Li Y, Chen J, Weng S, Yan C, Ye R, Zhu Y, et al. Hepatobiliary phase hypointensity on gadobenate dimeglumine-enhanced magnetic resonance imaging may improve the diagnosis of hepatocellular carcinoma. Ann Transl Med 2021;9:55. ArticlePubMedPMC
- 78. Braga FA, Torres US, Iared W, D Ippolito G. Does hypointense HCC in the hepatobiliary phase at gadoxetate-enhanced MRI predict recurrence after surgery? a systematic review and meta-analysis. Acad Radiol 2023;30:1298−1305.ArticlePubMed
- 79. Kitao A, Matsui O, Yoneda N, Kozaka K, Kobayashi S, Koda W, et al. Hypervascular hepatocellular carcinoma: correlation between biologic features and signal intensity on gadoxetic acid-enhanced MR images. Radiology 2012;265:780−789.ArticlePubMedPMC
- 80. Jang YR, Lee KW, Kim H, Lee JM, Yi NJ, Suh KS. Bile duct invasion can be an independent prognostic factor in early stage hepatocellular carcinoma. Korean J Hepatobiliary Pancreat Surg 2015;19:167−172.ArticlePubMedPMC
- 81. Shiomi M, Kamiya J, Nagino M, Uesaka K, Sano T, Hayakawa N, et al. Hepatocellular carcinoma with biliary tumor thrombi: aggressive operative approach after appropriate preoperative management. Surgery 2001;129:692−698.ArticlePubMed
- 82. Navadgi S, Chang CC, Bartlett A, McCall J, Pandanaboyana S. Systematic review and meta-analysis of outcomes after liver resection in patients with hepatocellular carcinoma (HCC) with and without bile duct thrombus. HPB (Oxford) 2016;18:312−316.ArticlePubMedPMC
- 83. Wu JY, Huang LM, Bai YN, Wu JY, Wei YG, Zhang ZB, et al. Imaging features of hepatocellular carcinoma with bile duct tumor thrombus: a multicenter study. Front Oncol 2021;11:723455. ArticlePubMedPMC
- 84. Qin LX, Ma ZC, Wu ZQ, Fan J, Zhou XD, Sun HC, et al. Diagnosis and surgical treatments of hepatocellular carcinoma with tumor thrombosis in bile duct: experience of 34 patients. World J Gastroenterol 2004;10:1397−1401.ArticlePubMedPMC
- 85. Zhou X, Wang J, Tang M, Huang M, Xu L, Peng Z, et al. Hepatocellular carcinoma with hilar bile duct tumor thrombus versus hilar cholangiocarcinoma on enhanced computed tomography: a diagnostic challenge. BMC Cancer 2020;20:54. ArticlePubMedPMCPDF
- 86. Jiang H, Wei J, Fu F, Wei H, Qin Y, Duan T, et al. Predicting microvascular invasion in hepatocellular carcinoma: a dualinstitution study on gadoxetate disodium-enhanced MRI. Liver Int 2022;42:1158−1172.PubMedPMC
- 87. Choi BI, Lee GK, Kim ST, Han MC. Mosaic pattern of encapsulated hepatocellular carcinoma: correlation of magnetic resonance imaging and pathology. Gastrointest Radiol 1990;15:238−240.ArticlePubMedPDF
- 88. Tohme S, Yazdani HO, Liu Y, Loughran P, van der Windt DJ, Huang H, et al. Hypoxia mediates mitochondrial biogenesis in hepatocellular carcinoma to promote tumor growth through HMGB1 and TLR9 interaction. Hepatology 2017;66:182−197.ArticlePubMedPMCPDF
- 89. Ling YH, Chen JW, Wen SH, Huang CY, Li P, Lu LH, et al. Tumor necrosis as a poor prognostic predictor on postoperative survival of patients with solitary small hepatocellular carcinoma. BMC Cancer 2020;20:607. ArticlePubMedPMCPDF
- 90. Wang Y, Zhang W, Ge H, Han X, Wu J, Sun X, et al. Tumor micronecrosis predicts poor prognosis of patients with hepatocellular carcinoma after liver transplantation. BMC Cancer 2023;23:86. ArticlePubMedPMCPDF
- 91. Wu ZJ, Xie YF, Chang X, Zhang L, Wu HY, Liu JB, et al. Type of necrosis influences prognosis in hepatocellular carcinoma after the first transarterial chemoembolization. Med Sci Monit 2021;27:e929884.ArticlePubMedPMC
- 92. Hong SB, Choi SH, Kim SY, Shim JH, Lee SS, Byun JH, et al. MRI features for predicting microvascular invasion of hepatocellular carcinoma: a systematic review and meta-analysis. Liver Cancer 2021;10:94−106.ArticlePubMedPMCPDF
- 93. Mori Y, Tamai H, Shingaki N, Hayami S, Ueno M, Maeda Y, et al. Hypointense hepatocellular carcinomas on apparent diffusion coefficient mapping: pathological features and metastatic recurrence after hepatectomy. Hepatol Res 2016;46:634−641.ArticlePubMed
- 94. Chang WC, Chen RC, Chou CT, Lin CY, Yu CY, Liu CH, et al. Histological grade of hepatocellular carcinoma correlates with arterial enhancement on gadoxetic acid-enhanced and diffusion-weighted MR images. Abdom Imaging 2014;39:1202−1212.ArticlePubMedPDF
- 95. Lee S, Kim SH, Hwang JA, Lee JE, Ha SY. Pre-operative ADC predicts early recurrence of HCC after curative resection. Eur Radiol 2019;29:1003−1012.ArticlePubMedPDF
- 96. Feo F, Pascale RM. Multifocal hepatocellular carcinoma: intrahepatic metastasis or multicentric carcinogenesis? Ann Transl Med 2015;3:4. PubMedPMC
- 97. Xie DY, Fan HK, Ren ZG, Fan J, Gao Q. Identifying clonal origin of multifocal hepatocellular carcinoma and its clinical implications. Clin Transl Gastroenterol 2019;10:e00006.ArticlePubMedPMC
- 98. Yue YY, Zhou WL. Hepatic resection is associated with improved long-term survival compared to radio-frequency ablation in patients with multifocal hepatocellular carcinoma. Front Oncol 2020;10:110. ArticlePubMedPMC
- 99. Risaliti M, Bartolini I, Campani C, Arena U, Xodo C, Adotti V, et al. Evaluating the best treatment for multifocal hepatocellular carcinoma: a propensity score-matched analysis. World J Gastroenterol 2022;28:3981−3993.ArticlePubMedPMC
- 100. Min JH, Lee MW, Park HS, Lee DH, Park HJ, Lim S, et al. Interobserver variability and diagnostic performance of gadoxetic acidenhanced MRI for predicting microvascular invasion in hepatocellular carcinoma. Radiology 2020;297:573−581.ArticlePubMed
- 101. Yoneda N, Matsui O, Kobayashi S, Kitao A, Kozaka K, Inoue D, et al. Current status of imaging biomarkers predicting the biological nature of hepatocellular carcinoma. Jpn J Radiol 2019;37:191−208.ArticlePubMedPDF
- 102. Ünal E, İdilman İS, Akata D, Özmen MN, Karçaaltıncaba M. Microvascular invasion in hepatocellular carcinoma. Diagn Interv Radiol 2016;22:125−132.ArticlePubMedPMC
- 103. Uenishi T, Kubo S, Yamamoto T, Shuto T, Ogawa M, Tanaka H, et al. Cytokeratin 19 expression in hepatocellular carcinoma predicts early postoperative recurrence. Cancer Sci 2003;94:851−857.ArticlePubMed
References
Figure & Data
References
Citations

 PubReader
PubReader ePub Link
ePub Link Download Citation
Download Citation
- Download Citation
- Close
- Related articles
-
- Changing etiology and epidemiology of hepatocellular carcinoma: Asia and worldwide
- Current perspectives on radiotherapy in hepatocellular carcinoma management: a comprehensive review
- Complications of immunotherapy in advanced hepatocellular carcinoma
- A multidisciplinary approach with immunotherapies for advanced hepatocellular carcinoma
- A single hepatic mass with two tales: hepatic tuberculosis and hepatocellular carcinoma

 E-submission
E-submission THE KOREAN LIVER CANCER ASSOCIATION
THE KOREAN LIVER CANCER ASSOCIATION


 Follow JLC on Twitter
Follow JLC on Twitter