Articles
- Page Path
- HOME > J Liver Cancer > Volume 19(1); 2019 > Article
-
Original Article
Discrepancy between the Actual Clinical Status of Patients with Hepatocellular Carcinoma and Expectations from Hepatocellular Carcinoma Surveillance: a Single-Center Study -
Nak Min Kim1, Young Seok Doh1
 , Ji Woong Jang1, Seok-Hwan Kim2, Hyuk Soo Eun3, Jae Hyuck Jun1, Sae Hee Kim1, Il Hyun Baek1, Sung Hee Jung1
, Ji Woong Jang1, Seok-Hwan Kim2, Hyuk Soo Eun3, Jae Hyuck Jun1, Sae Hee Kim1, Il Hyun Baek1, Sung Hee Jung1 -
Journal of Liver Cancer 2019;19(1):30-37.
DOI: https://doi.org/10.17998/jlc.19.1.30
Published online: March 31, 2019
1Department of Gastroenterology, Eulji University College of Medicine, Daejeon, Korea
2Division of Hepatology, Department of Internal Medicine, The Catholic University of Korea Daejeon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Daejeon, Korea
3Department of Internal Medicine, Chungnam National University School of Medicine, Daejeon, Korea
-
Corresponding author : Young Seok Doh Division of Gastroenterology, Department of Internal Medicine, Eulji University Hospital, Eulji University College of Medicine, 95 Dunsanseo-ro, Seo-Gu, Daejeon 35233, Korea
Tel. +82-42-611-3791, Fax. +82-42-259-1111 E-mail; elimier@gmail.com
• Received: December 22, 2018 • Revised: January 21, 2019 • Accepted: January 25, 2019
Copyright © 2019 The Korean Liver Cancer Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 4,422 Views
- 95 Downloads
- 2 Citations
Abstract
-
Background/Aims
- The National Liver Cancer Screening Program (NLCSP) has been implemented for the past 15 years in Korea. However, the actual clinical experience in Korea is inconsistent with the expectations of the hepatocellular carcinoma (HCC) surveillance program. To evaluate the actual clinical situation of HCC diagnoses, we investigated disease severity in patients with HCC and the diagnostic environment.
-
Methods
- From January 2011 to December 2015, all patients who were diagnosed with HCC in a single secondary hospital in Daejeon city were retrospectively enrolled in this study. Severity of HCC was evaluated according to the Barcelona Clinic Liver Cancer (BCLC) staging system.
-
Results
- Over the course of 5 years, 298 participants were enrolled. The mean age of participants was 64.0 years. Positive hepatitis B surface antigen was confirmed in 134 patients (45.0%), 35 patients (11.7%) tested positive for anti-hepatitis C virus antibody, and 93 patients (32.2%) had more than 40 g/day of alcohol consumption. The proportions of patients according to BCLC stages were as follows: BCLC-0, 28 patients (9.4%); BCLC-A, 42 patients (14.1%); BCLC-B, 26 patients (8.7%); BCLC-C, 134 patients (45.0%); and BCLC-D, 68 patients (22.8%). The diagnostic environments were as follows: 19 patients were in the NLCSP group (6.4%), 114 in the group with presenting signs (38.3%), 110 in the regular outpatient care group (36.9%), and 55 patients in the incidental diagnosis group (18.5%).
-
Conclusions
- Most patients (67.8%) had advanced stage HCC at diagnosis, and curative treatment was not indicated due to the severity disease. Thus, the actual situation is far worse than the theoretical expectation of HCC surveillance, suggesting that many high-risk patients for HCC are missed in surveillance.
- Hepatocellular carcinoma (HCC) is a major complication of liver disease that can lead to death. Methods of surveillance testing for HCC have been well established in many clinical studies [1-4]. Due to the low incidence of HCC, only liver sonography is recommended twice a year in Europe and the United States [5]. However, the combination of alpha-fetoprotein (AFP) analysis and liver sonography is recommended twice a year as an effective method for early diagnosis of HCC in Japan and Korea, where the incidence of HCC is high [6,7].
- For the past 16 years, the Ministry of Health and Welfare of Korea has mandated that all target populations participate in the National Liver Cancer Surveillance Program (NLCSP). The NLCSP offers free or low-cost surveillance testing (liver sonography and serum AFP) twice a year for high-risk patients with cirrhosis, chronic hepatitis B virus, or chronic hepatitis C virus (HCV) that has been documented during the previous 2 years, in the individual disease code data of the National Medical Insurance Corporation [8,9]. Before initiation (2003) of the NLCSP, 73.4% of patients were diagnosed with advanced stage HCC [10].
- A recent report showed encouraging results that most patients with chronic hepatitis B could be diagnosed at an early stage and treated radically for HCC [11]. However, many patients with recently diagnosed HCC are still diagnosed at advanced stages in the clinical setting, despite the expectations of HCC surveillance testing. Some patients are diagnosed after presenting with symptoms but have not undergone a regular check-up. Therefore, we aimed to investigate the clinical reality of HCC diagnosis during the past 5 years in Korea by reviewing medical records and examinations from a single secondary hospital in Daejeon city that conducts HCC diagnoses in patients who are referred from primary clinics.
INTRODUCTION
- 1. Participants
- From January 2011 to December 2015, all patients who were diagnosed with HCC at Eulji University Hospital in Daejeon were retrospectively enrolled in this study.
- 2. Diagnosing HCC
- According to the revised HCC guidelines in 2015 [12], the HCC diagnostic criteria are as follows. For patients with chronic hepatitis B, hepatitis C, and liver cirrhosis, tumor size larger than 1 cm and arterial phase enhancement and portal-delayed phase wash out, observed using dynamic computed tomography (CT) or dynamic magnetic resonance imaging (MRI), are diagnosed as HCC without performing a liver biopsy. If the tumor size is smaller than 1 cm, a continuous increase in AFP with controlled hepatitis and features of HCC demonstrated using dynamic CT and dynamic MRI result in a diagnosis of HCC. After identifying diagnoses of HCC, we collected patients’ demographic and life style information, test results, stage data, and information about the diagnostic environment.
- 3. Diagnostic environment
- We investigated medical records and request forms for patient referral in all participants diagnosed with HCC. We classified the diagnostic environment as follows: regular outpatient care (ROC), NLCSP, tumor or liver-related presenting signs (PS), and incidental diagnosis (ID) due to other medical conditions [13]. The ROC group included patients with results from laboratory testing and liver sonography that were performed at least once during the previous year. However, those surveillance methods do not satisfy the recommendations of the NLCSP and guidelines of the Korean Association for the Study of the Liver.
- For descriptive convenience, the NLCSP and ROC groups together comprised the regular surveillance (RS) group. Moreover, the PS and ID groups together comprised the group of omitted cases (OC) from surveillance testing.
- 4. HCC severity and staging
- The severity of HCC was evaluated according to the Barcelona Clinic Liver Cancer (BCLC) staging system, which is directly associated with proper treatment [14]. Tumor biology was evaluated on the basis of the size and number of tumors, infiltrative status based on images, vascular invasion, and serum AFP level.
- 5. Etiology of liver disease
- Chronic hepatitis B, chronic hepatitis C, and liver cirrhosis with any etiology are well known as important risk factors associated with development of HCC [3]. Chronic hepatitis B is the most common etiology of liver cirrhosis and HCC in Korea and most patients with chronic hepatitis B are aware of their disease. However, cirrhosis is often not diagnosed in patients with alcoholic liver cirrhosis owing to their lifestyle behaviors and lack of interest in their own health; however, it has been reported that of all the patients with liver cirrhosis in Korea, approximately 25-30% of the patients had alcoholic liver cirrhosis in Korea [15,16].
- Many of the participants in our study did not have a detailed profile of liver disease (HCV RNA titer qualitative test) and lacked follow-up records after their diagnosis of HCC because they were transferred to specialized hospitals immediately after they were diagnosed. Therefore, definite differentiation was limited by the retrospective study design and missing data.
- In this study, rather than using a diagnosis of chronic hepatitis B, we assessed HBsAg positivity. Additionally, rather than a diagnosis of chronic HCV, we assessed anti-HCV antibody (Ab) positivity. Alcoholic liver disease was defined according to alcohol intakes of greater than 20 g/day and 40 g/day [15].
- 6. Statistical methods
- Descriptive statistics were used to investigate the baseline characteristics of patients. The descriptive data are expressed as median and interquartile range. A t-test was used to compare continuous variables, and a Chi-square test was used to compare categorical variables. All analyses were performed using IBM SPSS ver. 18.0 (IBM Corp., Armonk, NY, USA), and we considered P <0.05 to indicate statistical significance.
METHODS
- A total of 298 patients diagnosed with HCC were enrolled in this study. The mean patient age at diagnosis was 64.0 years, and male patients were predominant (76.5%, 227 patients). At the time of diagnosis,134 patients (45.0%) had positive results for HBsAg and 35 patients (11.7%) were positive for anti-HCV Ab. The mean body mass index (BMI) among participants was 23.1 kg/m2, and 93 patients (32.2%) had daily alcohol intakes greater than 40 g. The distribution of Child-Pugh scores, used to assess chronic liver disease, was as follows: class A, 175 patients (58.7%); class B, 92 patients (30.9%); and class C, 31 patients (10.4%). Laboratory data at the time of HCC diagnosis are presented in Table 1.
- 1. Tumor size
- Considering the doubling time of HCC [3,5,6], the size of an HCC tumor is useful for estimating diagnostic delay. Single tumors smaller than 3 cm were found in 87 patients (30.1%). A total 95 patients (31.9%) had tumor size larger than 5 cm, and 69 patients (18.5%) had 4 or more tumors. Based on an abdominal CT, invasive-type HCC was found in 29 patients (9.7%) and 57 patients (19.1%) exhibited portal vein invasion (Table 2).
- 2. BCLC stages at diagnosis
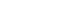
- According to BCLC staging, 28 patients (9.4%) had stage 0 HCC, 42 patients (14.1%) had stage A, 26 patients (8.7%) had stage B, 134 patients (45.0%) had stage C, and 68 patients (22.8%) had stage D (Fig. 1). Only 70 patients, accounting for 23.5% of the study participants, met the requirements for curative treatment (BCLC-0 or A). However, 202 patients, accounting for 67.8% of study participants, were indicated to undergo chemotherapy or supportive treatment due to advanced HCC (BCLC-C and D). The characteristics of patients with HCC according to BCLC stage are shown in Table 3.
- 3. Diagnostic environment
- There were several diagnostic pathways in the diagnosis of HCC. A total of 114 patients (38.3%) were diagnosed with tumor- or liver-related PS group. In addition, 110 patients (36.9%) were diagnosed in a setting of ROC, 19 patients (6.4%) were diagnosed via the NLCSP, and 55 patients (18.5%) were diagnosed as a result of ID during treatment for other medical conditions (ID group). Most patients were not diagnosed through RS testing (169 patients, 56.7%). Tumor- or liver-related symptoms included abdominal pain in 47 patients (tumor rupture in three patients), general weakness and weight loss in 11 patients, severe dyspepsia and nausea in two patients, gastrointestinal bleeding in 14 patients (hematemesis in nine patients), abdominal distension owing to ascites in 32 patients, jaundice in three patients, hepatic encephalopathy in four patients, and back pain with bone metastasis in one patient (Table 4).
- The diagnostic environment was analyzed according to severity of HCC. The diagnostic environments of patients with early-stage HCC (BCLC-0 and A) were as follows: one patient (1.4%) in the PS group (dyspepsia), 61 patients (87.1%) in the RS group, and eight patients (11.4%) in the ID group. The proportion of PS group increased with increasing disease severity. The rates of diagnosis as a result of RS testing were dramatically decreased (Fig. 2).
- Daily alcohol intake over 40 g and smoking showed a significantly high frequency in the OC group. Additionally, the prevalence of HBsAg positivity was significantly high in the RS group. Age, anti-HCV Ab positivity, and diabetes were not significantly correlated with either of these two diagnostic environments (OC and RS).
- 4. Characteristics of patients with advanced or terminal HCC
- Baseline characteristics of the 202 patients diagnosed with advanced or terminal HCC (BCLC-C and D) are as follows: mean age, 68 years; BMI, 22.6 kg/m2; HBsAg positive, 39.5%; anti-HCV Ab positive, 11.5%; and daily alcohol consumption over 40 g, 35.1%. Compared with the 70 patients diagnosed with early HCC (BCLC-0 or A), the significantly different baseline characteristics are as follows: the proportion of HBsAg-positive patients (P =0.003) and patient BMIs were significantly lower (P =0.003) in the group with advanced or terminal HCC; in addition, these patients had older age (P <0.001). The prevalence of excessive alcohol consumption was also significantly higher in the group with advanced or terminal HCC (P =0.013) than in the group with early-stage HCC.
- 5. Relatively young patients with HCC
- There were a number of relatively young patients who could not be screened in the current NLCSP system: five patients were younger than 40 years (three patients with hepatitis B and two with alcoholic liver cirrhosis). Of 12 patients under the age of 42 years at the time of diagnosis, three patients had alcoholic liver cirrhosis and nine had hepatitis B. Five of the nine patients with hepatitis B consumed more than 20 g of alcohol daily. There were no patients in this group with BCLC-0 and only two with BCLC-A; most (n=8) patients had advanced or terminal HCC with BCLC-C or D. Three patients each had tumor size 18 cm, 16 cm, and 8 cm. Only five patients had tumor sizes smaller than 3 cm.
- Based on the tumor sizes of the two 41-year-old patients (1.3 cm and 3.4 cm) and the five 42-year old patients (2 cm, 2.2 cm, 4.1 cm, 8 cm, and 12 cm), four patients (3.4 cm, 4.2 cm, 8 cm, 12 cm) could have developed HCC prior to the age of 40 years, considering the tumor doubling time [17].
RESULTS
- In Korea, the NLCSP has been implemented for the past 15 years in patients who are at high risk of developing HCC [8]. Based on the reports of several clinical studies, the surveillance program was expected to increase diagnosis of HCC in the early stages, to allow for curative treatment [1,13,18]. Recently, there have been encouraging reports that most patients with chronic hepatitis B were diagnosed in the early stage of HCC through a combination of semiannual liver ultrasonography and serum AFP testing [11]. However, in clinical practice, many patients are still diagnosed with advanced HCC, and related systematic evaluations and research into why this is the case have rarely been conducted.
- Before the NLCSP, only one study from the National Cancer Center, which is a tertiary hospital located in the Seoul area, reported that 24.8% of patients are diagnosed with HCC as a result of surveillance and 73.4% had advancedstage HCC [10]. However, there are no recent domestic research reports on the current diagnostic environment and severity of HCC, now that the NLCSP has been in place for 15 years.
- The NLCSP has increased rates of compliance with surveillance testing, from 38.5% in 2009 to 57.6% in 2017, although this remains at about half the target rate [19]. The NLCSP is intended for implementation among high-risk patients (those with chronic hepatitis B, chronic hepatitis C, and liver cirrhosis) who are aged 40 years or older, with disease information that has been documented during the previous 2 years, based on the National Health care Insurance Corporation data [8,20]. High-risk patients who have not received recent medical services or have delayed formal disease diagnosis are omitted from the NLCSP list [21]. Screening to identify the target high-risk population is limited to patients with alanine aminotransferase (ALT) levels over 46 IU/mL, so patients with normal ALT levels are not included in subsequent screening [20].
- In recent years, there has been increasing division between those hospitals in which patients with HCC are diagnosed and hospitals in which these patients are treated, and some data are missing as a result of patient referral. However, most patients with HCC are ultimately diagnosed at local secondary and tertiary hospitals in which three-phase abdominal CT, contrast-enhanced MRI, and liver biopsy are available. At Eulji University Hospital, one of the medical institutions that performs the final diagnoses of patients with HCC in Daejeon Metropolitan City and Chungcheongnam-do, all patients diagnosed with HCC from 2011 to 2015 were enrolled in this study. By minimizing selection bias, this study was designed to increase the reliability of assessment of the clinical reality of HCC diagnosis, with or without surveillance.
- In this study, 23.5% of the patients with HCC diagnosed in Daejeon and Chungchungnam-do had early-stage HCC (BCLC-0 and A), and these patients can be expected to have received curative treatment. Most patients (202, 67.8%) were diagnosed with advanced or terminal HCC (BCLC-C and D). This finding shows a large discrepancy with respect to the expectation [4].
- In particular, in terms of the type of diagnostic environment, surveillance tests are still not applied consistently among high-risk patients. A total 38.3% of patients (114 patients) were diagnosed as a result of tumor- or liver-related presenting signs (PS group) and not via surveillance. Additionally, 18.5% of patients (55 patients) were incidentally diagnosed (ID group). This means that many high-risk patients for HCC had been in a blind spot of surveillance and medical care.
- A previous study discussed the possibility that national cancer surveillance data are affected not only by overdiagnosis but also by omission because participants involved in the NLCSP are selected only on the basis of medical records from the previous 2 years in the National Health Insurance [9]. These omitted patients are estimated to be a high proportion, based on frequent clinical experiences with patients who had HCC but did not actually receive medical care and were often unaware of their liver disease.
- Even in the RS group (ROC and NLCSP), only 47.3% of patients had early-stage HCC, which is less than half, showing a discrepancy from the higher expected rate. This discrepancy from the expectation probably resulted from our restricting the ROC group to those patients with data from only one surveillance test, rather than the recommended two tests annually, documented in the year prior to diagnosis. The current system of surveillance twice a year was incorporated into the NLCSP in 2016. Before then, liver sonography and serum AFP testing once a year was implemented [9]. Thus, these changes could also affect the poor results of surveillance. In other aspects, the low compliance rate of 41-47% in the NLCSP can considerably explain the discrepancy, at least in the RS group [19].
- The low compliance rate of the NLCSP has limited statistical value because it is a result of poor access for individuals in the lower 50% with respect to socioeconomic level and health benefits. Compliance by the NLCSP group alone cannot explain all the patients missed in surveillance. First, there were a considerable number of patients who had never undergone surveillance, such as in the OC group. Second, even RS group including both of the NLCSP group and the ROC group accounted for only a small proportion (33.3%). Only massive absence of high-risk patients from surveillance can fully explain the approximately 40% rate of patients with HCC in the PS group, the low proportion (about one-third of all participants) in the ROC and NLCSP groups, and the relatively high proportion of patients with HCC in the ID group.
- Alcohol consumption was significantly higher in patients with advanced or terminal HCC than in those with earlystage HCC. This may be due to biological effects on tumor cells and the deterioration of liver function as a result of alcohol consumption. However, alcohol consumption was significantly higher in the OS group than in the RS group, suggesting the impact of patients’ neglect of their health and failure to undergo regular medical follow-up.
- As the severity of HCC increased, the HBsAg-positive rate decreased in this study. This assumes that patients with chronic hepatitis B understood their disease relatively well and participated in surveillance. The early diagnosis of infiltrative-type HCC tends to be difficult with current ultrasonography, so the proportion of patients with infiltrative-type HCC was higher in groups with advanced or terminal disease [22-25].
- The strength of this study lies in that it reflects real information about the clinical diagnostic environment and characteristics of patients with HCC upon diagnosis after the past 10 years of the NLCSP. Our study findings suggest that there is a blind spot in the health care system related to HCC. However, the number of participants was relatively small, and only single center data were analyzed. In particular, the retrospective study design restricted the investigation of detailed surveillance data and the individual problems of patients omitted from surveillance. A multicenter prospective study should be conducted to identify the socioeconomic situation and medical characteristics of patients who are missed by the current surveillance system.
- Efficient surveillance testing has been established in the diagnosis of HCC in Korea, but a discrepancy remains between the clinical reality and the expected result of surveillance. This suggests that even if a nationwide liver cancer surveillance program is implemented, major deficits would exist in the process of identifying high-risk patients and delivering medical resources. Therefore, further consideration must be given as to how to identify missed patients and implement the surveillance program for all high-risk patients, to yield the greatest benefit.
DISCUSSION
Figure 1.Severity of patients HCC at diagnosis, according to BCLC stage. HCC, hepatocellular carcinoma; BCLC, Barcelona Clinic Liver Cancer.
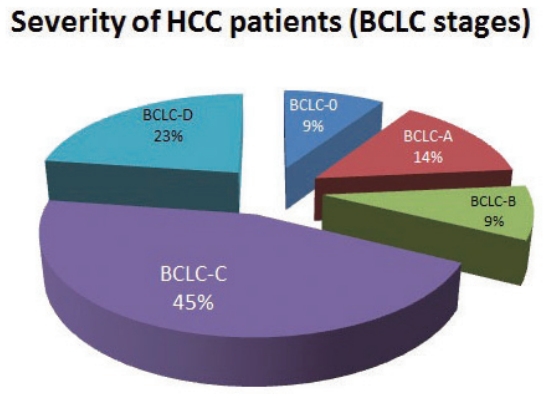

Figure 2.Proportion of diagnostic environment types in each BCLC stage. BCLC, Barcelona Clinic Liver Cancer.
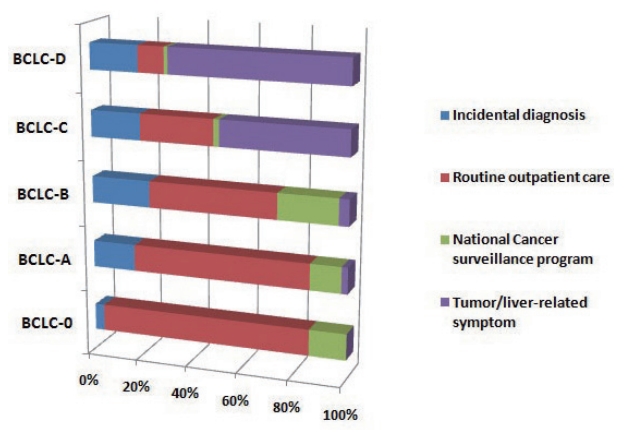

Table 1.Baseline characteristics of the enrolled subjects who were diagnosed with hepatocellular carcinoma
Table 2.Biological characteristics of the patients diagnosed with hepatocellular carcinoma
Table 3.Characteristics of the patients with hepatocellular carcinoma according to the BCLC stages
Table 4.Types of diagnostic environments of patients with hepatocellular carcinoma
- 1. Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol 2004;130:417−422.ArticlePubMed
- 2. Singal A, Volk ML, Waljee A, Salgia R, Higgins P, Rogers MA, et al. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther 2009;30:37−47.ArticlePubMedPMC
- 3. Zhao C, Nguyen MH. Hepatocellular carcinoma screening and surveillance: practice guidelines and real-life practice. J Clin Gastroenterol 2016;50:120−133.ArticlePubMed
- 4. Santi V, Trevisani F, Gramenzi A, Grignaschi A, Mirici-Cappa F, Del Poggio P, et al. Semiannual surveillance is superior to annual surveillance for the detection of early hepatocellular carcinoma and patient survival. J Hepatol 2010;53:291−297.ArticlePubMed
- 5. Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020−1022.ArticlePubMedPMC
- 6. Korean Liver Cancer Study Group (KLCSG), National Cancer Center, Korea (NCC). 2014 KLCSG-NCC Korea practice guideline for the management of hepatocellular carcinoma. Gut Liver 2015;9:267−317.PubMedPMC
- 7. Kokudo N, Makuuchi M. Evidence-based clinical practice guidelines for hepatocellular carcinoma in Japan: the J-HCC guidelines. J Gastroenterol 2009;44 Suppl 19:119−121.ArticlePubMedPDF
- 8. Lee EH, Han MA, Lee HY, Jun JK, Choi KS, Park EC. Liver cancer screening in Korea: a report on the 2008 national cancer screening programme. Asian Pac J Cancer Prev 2010;11:1305−1310.PubMed
- 9. Shim JJ, Park HJ, Kim JW, Hwang EJ, Lee CK, Jang JY, et al. The Korean national liver cancer surveillance program: experience of a single healthcare center in 2011. Korean J Med 2013;84:672−680.Article
- 10. Cheon JH, Park JW, Park KW, Kim YI, Kim SH, Lee WJ, et al. The clinical report of 1,078 cases of hepatocellular carcinomas: national cancer center experience. Korean J Hepatol 2004;10:288−297.PubMed
- 11. Choi IS, Oh CH, Park SY, Ahn SE, Park SJ, Choi HR, et al. Incidence of primary liver cancer in subjects with chronic hepatitis B in Korean national liver cancer screening program. J Liver Cancer 2017;17:136−143.Article
- 12. Kim DY, Kim HJ, Jeong SE, Kim SG, Kim HJ, Sinn DH, et al. The Korean guideline for hepatocellular carcinoma surveillance. J Korean Med Assoc 2015;58:385−397.Article
- 13. Trevisani F, Cantarini MC, Labate AM, De Notariis S, Rapaccini G, Farinati F, et al. Surveillance for hepatocellular carcinoma in elderly Italian patients with cirrhosis: effects on cancer staging and patient survival. Am J Gastroenterol 2004;99:1470−1476.ArticlePubMed
- 14. European Association For The Study Of The Liver, European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908−943.ArticlePubMed
- 15. Yoon YS, Oh SW, Baik HW, Park HS, Kim WY. Alcohol consumption and the metabolic syndrome in Korean adults: the 1998 Korean national health and nutrition examination survey. Am J Clin Nutr 2004;80:217−224.ArticlePubMedPDF
- 16. Han YS, Kim BH, Baek IY, Lee DK, Kim KJ, Dong SH, et al. The change of the etiology, complications and cause of death of the liver cirrhosis in 1990s. Korean J Hepatol 2000;6:328−339.
- 17. Kubota K, Ina H, Okada Y, Irie T. Growth rate of primary single hepatocellular carcinoma: determining optimal screening interval with contrast enhanced computed tomography. Dig Dis Sci 2003;48:581−586.ArticlePubMed
- 18. Yoo KY, Kim H, Lee MS, Park BJ, Ahn YO, Lee HS, et al. A reconstructed cohort study on the hepatitis B virus infection as a risk factor of liver cancer in Korea. J Korean Med Sci 1991;6:319−324.ArticlePubMedPMC
- 19. Ministry of Health and Welfare, Korea Central Cancer Registry, Department of Disease Policy. Examination rate of national cancer screening program in Korea in 2018 [Internet]. Wonju (KR): National Health Insurance Service; [cited 2019 Dec 01]. Available from: http://www.index.go.kr/potal/main/EachDtlPageDetail.do?idx_cd=1440
- 20. Ministry of Health and Welfare. Guideline of national cancer screening program 2011. Seoul, Ministry of Health and Welfare. 2011.
- 21. An C, Choi YA, Choi D, Paik YH, Ahn SH, Kim MJ, et al. Growth rate of early-stage hepatocellular carcinoma in patients with chronic liver disease. Clin Mol Hepatol 2015;21:279−286.ArticlePubMedPMC
- 22. Yu NC, Chaudhari V, Raman SS, Lassman C, Tong MJ, Busuttil RW, et al. CT and MRI improve detection of hepatocellular carcinoma, compared with ultrasound alone, in patients with cirrhosis. Clin Gastroenterol Hepatol 2011;9:161−167.ArticlePubMed
- 23. Sinn DH, Yi J, Choi MS, Choi D, Gwak GY, Paik YH, et al. Incidence and risk factors for surveillance failure in patients with regular hepatocellular carcinoma surveillance. Hepatol Int 2013;7:1010−1018.ArticlePubMed
- 24. Myung SJ, Yoon JH, Kim KM, Gwak GY, Kim YJ, Yu JW, et al. Diffuse infiltrative hepatocellular carcinomas in a hepatitis B-endemic area: diagnostic and therapeutic impediments. Hepatogastroenterology 2006;53:266−270.PubMed
- 25. Seror O, N’Kontchou G, Haddar D, Dordea M, Ajavon Y, Ganne N, et al. Large infiltrative hepatocellular carcinomas: treatment with percutaneous intraarterial ethanol injection alone or in combination with conventional percutaneous ethanol injection. Radiology 2005;234:299−309.ArticlePubMed
References
Figure & Data
References
Citations
Citations to this article as recorded by 

- NCA‐GA‐SVM: A new two‐level feature selection method based on neighborhood component analysis and genetic algorithm in hepatocellular carcinoma fatality prognosis
Wojciech Książek, Filip Turza, Paweł Pławiak
International Journal for Numerical Methods in Biomedical Engineering.2022;[Epub] CrossRef - Imaging Modalities for Hepatocellular Carcinoma Surveillance: Expanding Horizons beyond Ultrasound
Hyo Jung Park, So Yeon Kim
Journal of Liver Cancer.2020; 20(2): 99. CrossRef

 E-submission
E-submission THE KOREAN LIVER CANCER ASSOCIATION
THE KOREAN LIVER CANCER ASSOCIATION
 PubReader
PubReader ePub Link
ePub Link Download Citation
Download Citation

 Follow JLC on Twitter
Follow JLC on Twitter