Search
- Page Path
- HOME > Search
Original Articles
- Comparison of atezolizumab plus bevacizumab and lenvatinib for hepatocellular carcinoma with portal vein tumor thrombosis
- Jeayeon Park, Yun Bin Lee, Yunmi Ko, Youngsu Park, Hyunjae Shin, Moon Haeng Hur, Min Kyung Park, Dae-Won Lee, Eun Ju Cho, Kyung-Hun Lee, Jeong-Hoon Lee, Su Jong Yu, Tae-Yong Kim, Yoon Jun Kim, Tae-You Kim, Jung-Hwan Yoon
- J Liver Cancer. 2024;24(1):81-91. Published online January 19, 2024
- DOI: https://doi.org/10.17998/jlc.2023.12.25
- 1,082 Views
- 136 Downloads
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background/Aim
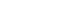
Atezolizumab plus bevacizumab and lenvatinib are currently available as first-line therapy for the treatment of unresectable hepatocellular carcinoma (HCC). However, comparative efficacy studies are still limited. This study aimed to investigate the effectiveness of these treatments in HCC patients with portal vein tumor thrombosis (PVTT).
Methods
We retrospectively included patients who received either atezolizumab plus bevacizumab or lenvatinib as first-line systemic therapy for HCC with PVTT. Primary endpoint was overall survival (OS), and secondary endpoints included progressionfree survival (PFS) and disease control rate (DCR) determined by response evaluation criteria in solid tumors, version 1.1.
Results
A total of 52 patients were included: 30 received atezolizumab plus bevacizumab and 22 received lenvatinib. The median follow-up duration was 6.4 months (interquartile range, 3.9-9.8). The median OS was 10.8 months (95% confidence interval [CI], 5.7 to not estimated) with atezolizumab plus bevacizumab and 5.8 months (95% CI, 4.8 to not estimated) with lenvatinib (P=0.26 by log-rank test). There was no statistically significant difference in OS (adjusted hazard ratio [aHR], 0.71; 95% CI, 0.34-1.49; P=0.37). The median PFS was similar (P=0.63 by log-rank test), with 4.1 months (95% CI, 3.3-7.7) for atezolizumab plus bevacizumab and 4.3 months (95% CI, 2.6-5.8) for lenvatinib (aHR, 0.93; 95% CI, 0.51-1.69; P=0.80). HRs were similar after inverse probability treatment weighting. The DCRs were 23.3% and 18.2% in patients receiving atezolizumab plus bevacizumab and lenvatinib, respectively (P=0.74).
Conclusion
The effectiveness of atezolizumab plus bevacizumab and lenvatinib was comparable for the treatment of HCC with PVTT.

- The effects of immune checkpoint modulators on the clinical course of patients with resectable hepatocellular carcinoma
- Jihyun An, Hyo Jeong Kang, Eunsil Yu, Han Chu Lee, Ju Hyun Shim
- J Liver Cancer. 2022;22(1):40-50. Published online March 17, 2022
- DOI: https://doi.org/10.17998/jlc.2022.03.06

- 3,365 Views
- 113 Downloads
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background/Aim
Immune checkpoint proteins regulating T-cell mediated anti-tumor immunity have been reported to affect clinical outcomes in multiple malignancies. This study aimed to investigate the prognostic effect of histological expression of immune checkpoint proteins in patients with resected hepatocellular carcinoma (HCC).
Methods
A total of 221 patients with HCC who underwent curative resection were included. Expression of programmed-cell death ligand-1 (PD-L1) in tumor cells (tPD-L1) and tumor infiltrating mononuclear cells (TIMCs) (iPD-L1), programmed-cell death-1 in TIMCs (iPD-1), and cytotoxic T lymphocyte antigen-4 in TIMCs (iCTLA-4) were measured immunohistochemically.
Results
Histo-positivity for iCTLA-4, iPD-1, iPD-L1, and tPD-L1 was 32.1%, 42.5%, 35.3%, and 14.9%, respectively. Multivariate logistic analyses revealed that male sex and tumor >5 cm were variables related to iCTLA-4 positivity (odds ratio [OR], 0.46 and 1.94, respectively; P<0.05). Poor differentiation was related to PD-L1 expression in both tumor cells and TIMCs (OR, 2.88 and 3.46, respectively; P<0.05). Microvascular invasion was significantly associated only with iPD-L1 (OR, 2.24; P<0.05). In time-dependent outcome analyses, expression of immune checkpoint proteins in TIMCs (i.e., iCTLA-4, iPD-1, and iPD-L1) was significantly related to longer overall survival and non-cancer-related survival (all P<0.05), but not to time-to-recurrence or cancer-specific deaths. Concurrent activation of the PD-1:PD-L1 and CTLA-4 pathways predicted improved outcomes in terms of overall survival and non-cancer related survival (P=0.06 and P=0.03, respectively).
Conclusions
Immune checkpoint proteins upregulated in TIMCs in HCC tissues have individual and additive effects in prolonging the survival of patients, specifically in terms of survival not related to cancer recurrence.

Review Article
- Advances in immune checkpoint inhibitors for hepatocellular carcinoma
- Ji Won Han, Su-Hyung Park
- J Liver Cancer. 2021;21(2):139-145. Published online September 30, 2021
- DOI: https://doi.org/10.17998/jlc.2021.09.24

- 3,605 Views
- 100 Downloads
- 3 Citations
-
 Abstract
Abstract
 PDF
PDF - Hepatocellular carcinoma (HCC) is the fifth most common cancer, and the second leading cause of cancer-related death worldwide. Although recent advances in immune checkpoint inhibitor-based immunotherapy have initiated a new era for advanced HCC treatment, the majority of HCC patients receiving immune checkpoint blockades do not derive clinical benefit. Thus, there remains an urgent need for novel immunotherapeutic strategies with improved therapeutic efficacy. Here we review recent studies of immune checkpoint blockade in HCC, providing the necessary basis for the rational design of immunotherapy.
-
Citations
Citations to this article as recorded by- Systematic Review of Molecular Targeted Therapies for Adult-Type Diffuse Glioma: An Analysis of Clinical and Laboratory Studies
Logan Muzyka, Nicolas K. Goff, Nikita Choudhary, Michael T. Koltz
International Journal of Molecular Sciences.2023; 24(13): 10456. CrossRef - Integrative analysis of lactylation-related genes and establishment of a novel prognostic signature for hepatocellular carcinoma
Diankui Cai, Xiaoqing Yuan, D. Q. Cai, Ang Li, Sijia Yang, Weibang Yang, Jinxin Duan, Wenfeng Zhuo, Jun Min, Li Peng, Jinxing Wei
Journal of Cancer Research and Clinical Oncology.2023; 149(13): 11517. CrossRef - Editorial on immune checkpoint inhibitors in the treatment of hepatocellular carcinoma
Samantha M Ruff, Timothy M Pawlik
Immunotherapy.2023; 15(16): 1323. CrossRef
- Systematic Review of Molecular Targeted Therapies for Adult-Type Diffuse Glioma: An Analysis of Clinical and Laboratory Studies

Original Article
- Infiltration of T Cells and Programmed Cell Death Ligand 1-expressing Macrophages as a Potential Predictor of Lenvatinib Response in Hepatocellular Carcinoma
- Pil Soo Sung, Sung Woo Cho, Jaejun Lee, Hyun Yang, Jeong Won Jang, Si Hyun Bae, Jong Young Choi, Seung Kew Yoon
- J Liver Cancer. 2020;20(2):128-134. Published online September 30, 2020
- DOI: https://doi.org/10.17998/jlc.20.2.128
- 3,287 Views
- 98 Downloads
- 6 Citations
-
 Abstract
Abstract
 PDF
PDF - Background/Aim
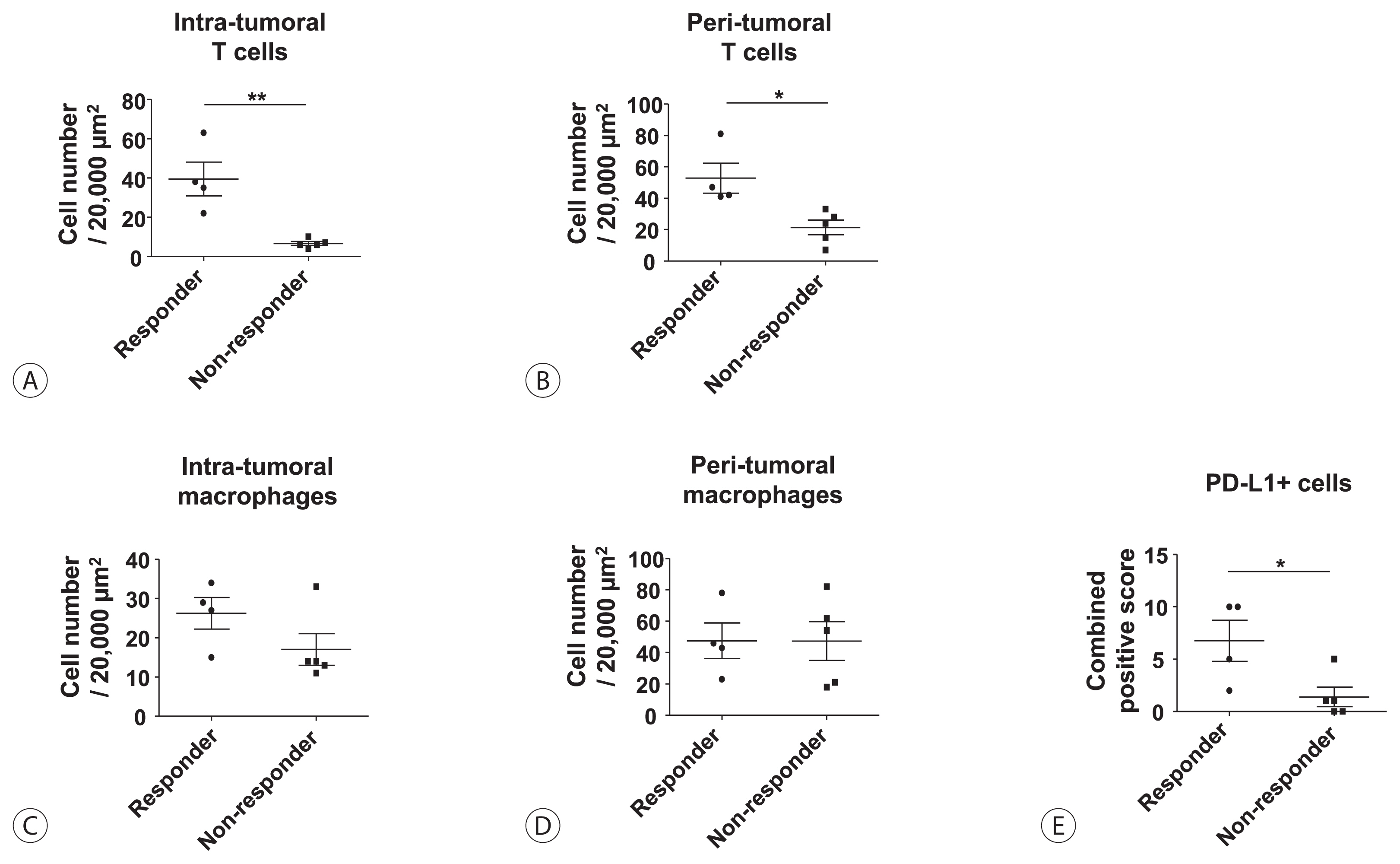
s: Lenvatinib was recently proven to be non-inferior to sorafenib in treating unresectable hepatocellular carcinoma (HCC) in a phase-3 randomized controlled trial. In this study, we investigated whether the response to lenvatinib was affected by tumor immunogenicity.
Methods
Between May 2019 and April 2020, nine patients with intermediate-to-advanced HCC, who were treated with lenvatinib after liver biopsy, were enrolled. Immunohistochemical staining and multi-color flow cytometry were performed on specimens obtained from liver biopsy.
Results
Among the nine patients enrolled, four showed objective responses (complete responses+partial responses). Immunohistochemical staining for CD3, CD68, and programmed cell death ligand 1 (PD-L1) demonstrated that patients with objective responses showed marked infiltration of T cells and PD-L1-expressing macrophages in intra-tumoral and peri-tumoral tissues compared to those without objective responses. A significant difference in the numbers of infiltrated T cells, both in the intra-tumoral (P<0.01) and peri-tumoral regions (P<0.05), were identified between responders and non-responders. Regarding the number of infiltrated macrophages, no significant difference was found between the responders and non-responders, although the number of PD-L1-expressing tumor-associated macrophages was significantly higher in responders than that in non-responders (P<0.05).
Conclusions
Tumor immunogenicity, as indicated by T cell and PD-L1-positive macrophage infiltration, affects lenvatinib response in unresectable HCC. -
Citations
Citations to this article as recorded by- Higher Number of Tumor-Infiltrating PD-L1+ Cells Is Related to Better Response to Multikinase Inhibitors in Hepatocellular Carcinoma
Ji Won Han, Ji Hoon Kim, Dong Hyun Kim, Jeong Won Jang, Si Hyun Bae, Jong Young Choi, Seung Kew Yoon, Jaegyoon Ahn, Hyun Yang, Pil Soo Sung
Diagnostics.2023; 13(8): 1453. CrossRef - Intrahepatic inflammatory IgA+PD-L1high monocytes in hepatocellular carcinoma development and immunotherapy
Pil Soo Sung, Dong Jun Park, Pu Reun Roh, Kyoung Do Mun, Sung Woo Cho, Gil Won Lee, Eun Sun Jung, Sung Hak Lee, Jeong Won Jang, Si Hyun Bae, Jong Young Choi, Jonghwan Choi, Jaegyoon Ahn, Seung Kew Yoon
Journal for ImmunoTherapy of Cancer.2022; 10(5): e003618. CrossRef - Crosstalk between tumor-associated macrophages and neighboring cells in hepatocellular carcinoma
Pil Soo Sung
Clinical and Molecular Hepatology.2022; 28(3): 333. CrossRef - Blood-based biomarkers for immune-based therapy in advanced HCC: Promising but a long way to go
Pil Soo Sung, Isaac Kise Lee, Pu Reun Roh, Min Woo Kang, Jaegyoon Ahn, Seung Kew Yoon
Frontiers in Oncology.2022;[Epub] CrossRef - Immunological Mechanisms for Hepatocellular Carcinoma Risk after Direct-Acting Antiviral Treatment of Hepatitis C Virus Infection
Pil Soo Sung, Eui-Cheol Shin
Journal of Clinical Medicine.2021; 10(2): 221. CrossRef - Preferential Expression of Programmed Death Ligand 1 Protein in Tumor-Associated Macrophages and Its Potential Role in Immunotherapy for Hepatocellular Carcinoma
Dong-Jun Park, Pil-Soo Sung, Gil-Won Lee, Sung-Woo Cho, Sung-Min Kim, Byung-Yoon Kang, Won-Hee Hur, Hyun Yang, Soon-Kyu Lee, Sung-Hak Lee, Eun-Sun Jung, Chang-Ho Seo, Joseph Ahn, Ho-Joong Choi, Young-Kyoung You, Jeong-Won Jang, Si-Hyun Bae, Jong-Young Cho
International Journal of Molecular Sciences.2021; 22(9): 4710. CrossRef
- Higher Number of Tumor-Infiltrating PD-L1+ Cells Is Related to Better Response to Multikinase Inhibitors in Hepatocellular Carcinoma


 E-submission
E-submission THE KOREAN LIVER CANCER ASSOCIATION
THE KOREAN LIVER CANCER ASSOCIATION

 First
First Prev
Prev



 Follow JLC on Twitter
Follow JLC on Twitter